Session Information
Session Type: Abstract Session
Session Time: 10:00AM-10:50AM
Background/Purpose: Vagus nerve stimulation (VNS) has been shown to improve rheumatoid arthritis (RA) disease severity in patients with refractory disease (1,2). A multi-center pilot study investigated the safety and efficacy of a wearable device to treat RA via electrical stimulation of the auricular branch of the vagus nerve.
Methods: Patients with active RA defined by ≥4 tender/swollen joints (28 jt count), DAS28-CRP >3.8, active synovitis detected on ultrasound and MRI, and who had inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) were enrolled in the open-label pilot study. The primary endpoint was the change in the DAS28-CRP score at Week 12. Secondary endpoints included the mean change in HAQ-DI, the proportion of patients achieving ACR20/50/70, and the proportion of patients achieving a HAQ-DI of 0.22. Low disease activity (LDA) and remission were assessed based on DAS28-CRP (LDA if < 3.2; remission if < 2.6). MRI was performed at Weeks 0 and 12 and changes in synovitis, osteitis and bone erosion were assessed using the OMERACT-RAMRIS method. Safety and adverse events were assessed throughout the study.
Results: Thirty patients were enrolled and 27 completed the study. The mean age was 54 years, 90% were female, and the mean duration of disease was 7.3 years. The mean change in DAS28-CRP at Week 12 was -1.4 (p< 0.001) (Figure 1). Eleven patients (37%) attained LDA and 7 (23%) achieved remission. The mean percent change in the DAS28-CRP at Week 12 for patients with moderate and high disease activity was 24% and 27% respectively (Figure 2). ACR20/50/70 response rates were 53%, 33%, and 17% respectively. Individual components of DAS28-CRP and ACR scoring for all observed data appear in Table 1. The change in HAQ-DI from baseline was -0.47 (p< 0.05), and 57% of patients achieved an overall HAQ-DI reduction of 0.22 or more. The mean change in the OMERACT-RAMRIS synovitis, osteitis, and bone erosion scores at week 12 compared to baseline were -0.10 (p=0.16), -0.43 (p=0.46), and 0.047 (p=0.80), respectively. Four adverse events (AEs) were reported: 1 device-related AE due to a superficial skin abrasion where the earpiece contacts the ear and 3 non-device related AEs -- 2 accidental patient falls and one report of mucous build-up in the throat. All 4 AEs resolved without intervention or further sequelae.
Conclusion: In this pilot study, transcutaneous stimulation of the auricular branch of the vagus nerve was well tolerated and improved signs and symptoms of RA. Further evaluation in larger controlled studies is needed to confirm whether this non-invasive vagus nerve stimulator might offer an alternative approach to the treatment of RA.
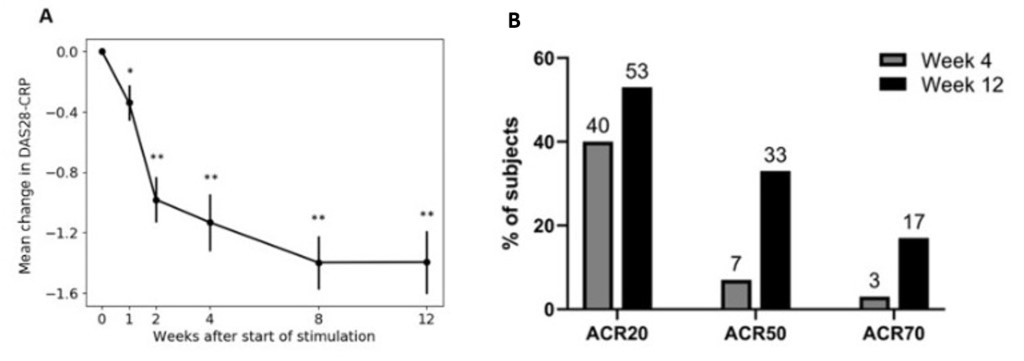 Figure 1: Primary and secondary endpoint. A) Mean change in DAS28-CRP score for each study visit. For the 27 patients who completed the study, there is a significant reduction in the DAS28-CRP after Week 1, and it continues to decrease through the end of the study. At Week 12, there is a mean reduction of 1.40 in the DAS28-CRP relative to Baseline. B) Percentage of all 30 patients that achieved ACR20/50/70 at Week 4 (grey) and Week 12 (black). The percentage of patients who met ACR20/50/70 is provided at the top of each bar. Patients who did not complete the study were deemed non-responders. One (*) asterisk indicates p < 0.05 and two (**) asterisks indicate p < 0.01. P-values were calculated using paired two-tail t-tests.
Figure 1: Primary and secondary endpoint. A) Mean change in DAS28-CRP score for each study visit. For the 27 patients who completed the study, there is a significant reduction in the DAS28-CRP after Week 1, and it continues to decrease through the end of the study. At Week 12, there is a mean reduction of 1.40 in the DAS28-CRP relative to Baseline. B) Percentage of all 30 patients that achieved ACR20/50/70 at Week 4 (grey) and Week 12 (black). The percentage of patients who met ACR20/50/70 is provided at the top of each bar. Patients who did not complete the study were deemed non-responders. One (*) asterisk indicates p < 0.05 and two (**) asterisks indicate p < 0.01. P-values were calculated using paired two-tail t-tests.
 Figure 2: The mean change in DAS28-CRP score for patients with moderate or high disease activity at Baseline. The DAS28-CRP percentage change from Baseline is provided at the bottom of each bar.
Figure 2: The mean change in DAS28-CRP score for patients with moderate or high disease activity at Baseline. The DAS28-CRP percentage change from Baseline is provided at the bottom of each bar.
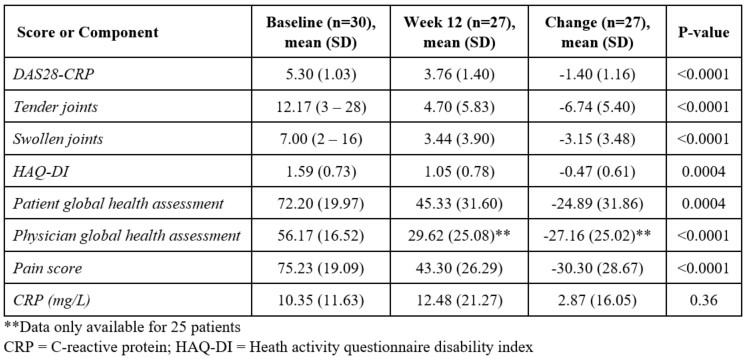 Table 1: Individual components of DAS28-CRP and ACR scoring for all observed data.
Table 1: Individual components of DAS28-CRP and ACR scoring for all observed data.
To cite this abstract in AMA style:
Marsal S, Corominas H, De Agustin De Oro J, Perez Garcia C, Lopez Lasanta M, Borrell H, Reina D, Sanmartí R, Narváez F, Franco-Jarava C, Peterfy C, Narvaez J, Sharma V, Alataris K, Genovese M, Baker M. Non-invasive Vagus Nerve Stimulation Improves Signs and Symptoms of Rheumatoid Arthritis: Results of a Pilot Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/non-invasive-vagus-nerve-stimulation-improves-signs-and-symptoms-of-rheumatoid-arthritis-results-of-a-pilot-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/non-invasive-vagus-nerve-stimulation-improves-signs-and-symptoms-of-rheumatoid-arthritis-results-of-a-pilot-study/
