Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Emperipolesis (EP) is a little-understood phenomenon referring to the presence neutrophils within megakaryocytes (MKs). We developed methods to study EP in vitro and in vivo, finding that EP is a highly regulated cell-in-cell interaction, requires cytoskeleton rearrangement by neutrophils and MKs, and results in the reciprocal exchange of cell membrane and proteins between both lineages (Cunin P et al. eLife 2019;8:e44031). At the end of EP, neutrophils exit MK cytoplasm alive and intact, but the consequences for neutrophil function are unknown
Methods: We assessed the frequency of EP in healthy mice, in the K/BxN mouse model of inflammatory arthritis and in the cecal ligation and puncture model. A model of EP was developed through incubation of MKs together with neutrophils and characterized using confocal imaging and electron microscopy. The biology of EP was interrogated in vitro by time-lapse microscopy on µ-slide chemotaxis chambers, and in vivo using whole-mount immunofluorescence of bone marrow. The role of EP on neutrophil migration was investigated in vitro using transwell assay and in vivo through engraftment of labelled neutrophils co-cultured or not with MKs back into mice subjected to inflammation
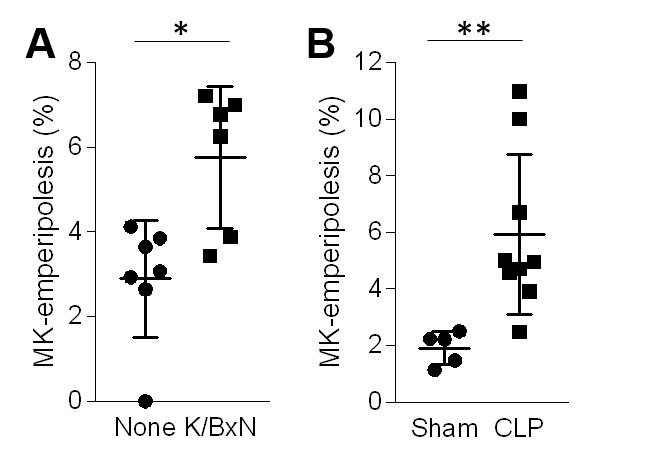
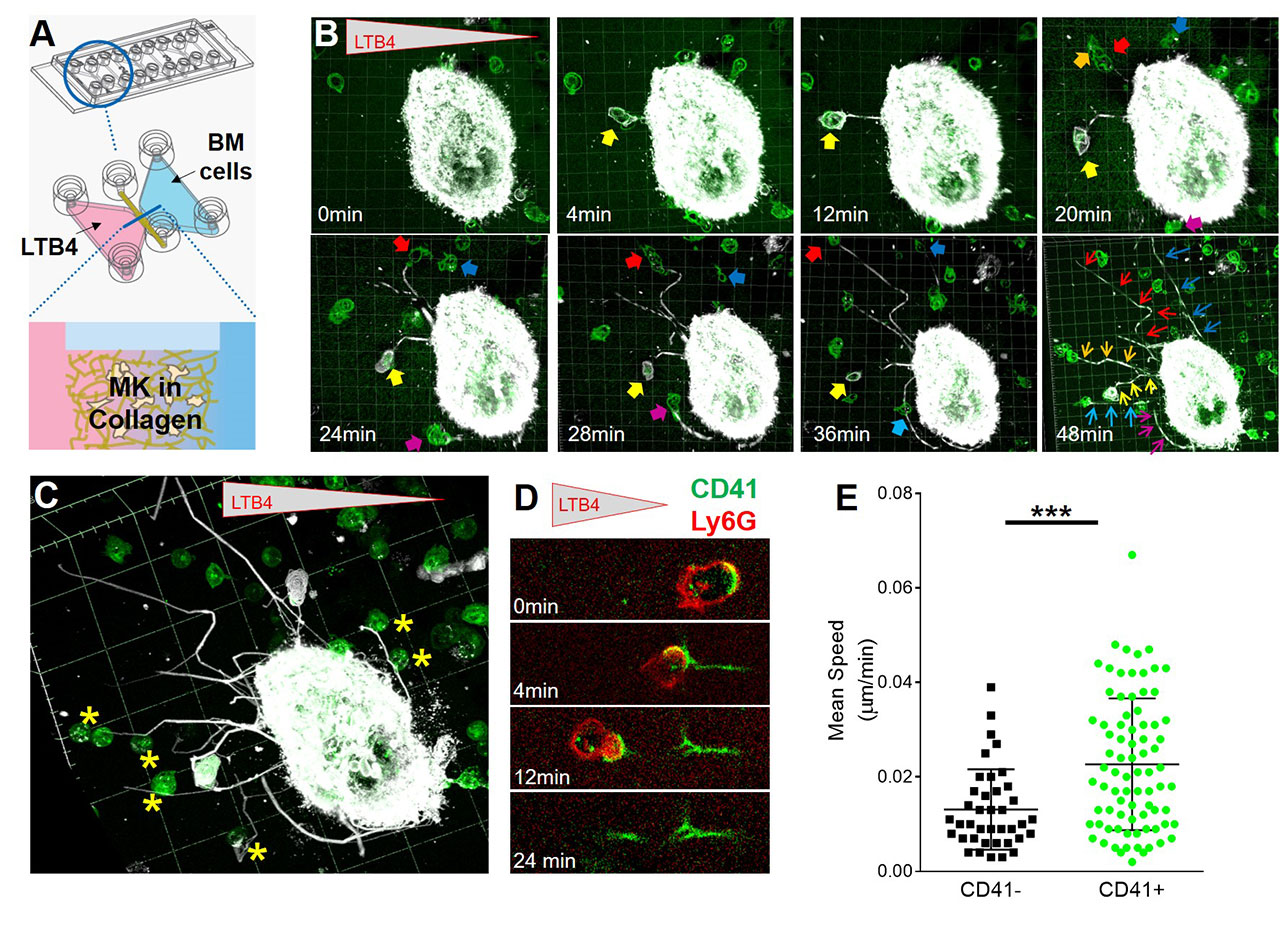
Results: Histological sections of bone marrow revealed that EP is increased 2-3 fold in inflammatory arthritis and after cecal ligation and puncture, a model of polymicrobial sepsis (Figure 1). Whole-mount immunostaining in bone marrow showed that EP is restricted to MKs in contact with vascular sinusoids, and neutrophils can be observed in the space between MKs and blood vessels, suggesting egress directly into blood (Figure 2). In vitro, we observed that neutrophils enter MKs through a membrane-lined vacuole, termed “emperisome”. We routinely noted the appearance of MK-derived exosomes in the emperisome, and the uptake of MK exosomes by internalized neutrophils. Migration assay on µ-slide chemotaxis chambers revealed that neutrophils enter and exit MKs following a chemotactic gradient, using a “trans-MK” route (Figure 3A-B). After their passage into MK, neutrophils deposit MK material behind them during their migration, forming a “trail”-like structure made of MK proteins involved in immune cell attachment, and these neutrophils exhibit a clear migratory advantage (Figure 3B-E and not shown). In transwell assay, we observed that neutrophils previously co-cultured with MKs migrate more efficiently toward an LTB4 gradient. Neutrophil migration is not modulated after culture with MK supernatant or PFA-fixed MKs, excluding an effect from MK-derived microparticles, cytokines, or surface proteins and suggesting a role for EP. In vivo, neutrophils previously incubated with MKs migrated more efficiently into the peritoneal cavity during LPS- or IL-1β-induced peritonitis
Conclusion: EP is dramatically increased during inflammation and mediates transfer of membranes and MK-exosome contents to internalized neutrophils. 3-D bone marrow microscopy and migration assays suggest that neutrophils can exit from MKs directly into the circulation, resulting in enhanced migratory capacity. Additional consequences of EP for neutrophil biology remain to be defined
« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neutrophil-passage-through-the-megakaryocyte-cytoplasm-via-emperipolesis-modulates-neutrophil-migration/