Session Information
Date: Sunday, October 26, 2025
Title: (0210–0232) Measures & Measurement of Healthcare Quality Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Allopurinol can cause life-threatening severe cutaneous adverse reactions, especially in patients who carry the HLA-B58:01 allele. Because this allele is more common among Southeast Asian and African American individuals, the 2020 American College of Rheumatology Gout Guidelines conditionally recommend genetic testing for these groups before starting allopurinol. To support safer prescribing, we developed and evaluated a national electronic dashboard to promote HLA-B58:01 testing among at-risk allopurinol users in the U.S. Veterans Health Administration (VHA).
Methods: We identified allopurinol users in the VHA using data from the Corporate Data Warehouse. A dashboard was built using Microsoft PowerBI to display patient identifiers, HLA-B58:01 testing status, and self-reported race/ethnicity (Figure 1). The dashboard launched nationally in April 2023, and all VHA rheumatologists were notified by email. Dashboard use at each facility was tracked through PowerBI activity logs. Facilities were excluded if they were pilot sites (n=6), used a different EHR (Cerner; n=6), lacked structured fields for HLA-B58:01 testing (n=50), or had no recorded dashboard sessions (n=11).The main outcome was the weekly percentage of allopurinol users with self-reported Asian or African American race who had completed HLA-B*58:01 testing, between October 2022 and April 2023 (pre-launch) and May 2023 to April 2024 (post-launch). Facilities were categorized as high or low engagement based on how often they used the dashboard after launch (≥20% of weeks vs. < 20%). We used a two-group interrupted time series (ITS) analysis to compare trends in testing between high- and low-engagement facilities.
Results: The analysis included 57 VHA facilities (86% classified as high complexity), serving 45,500 at-risk allopurinol users. Patients were 97% male, with a mean age of 67.3 years; 88.5% were African American and 11.5% were Asian. Overall, HLA-B*58:01 testing increased from 1.6% at baseline to 3.0% at the end of the study (Table). ITS analysis showed a significantly faster increase in testing at high-engagement sites compared to low-engagement sites (post-intervention trend difference: +0.024 percentage points/week, 95% CI: 0.002–0.047; p = 0.03; Figure 2).
Conclusion: Although overall testing rates remained low, implementation of a national dashboard was associated with modest but significant increases in HLA-B*58:01 testing—especially at facilities that regularly used the tool. Future studies should assess testing in an incident user cohort of patients. Ongoing work should focus on maintaining clinician engagement, improving dashboard usability, and assessing the long-term impact on patient safety.
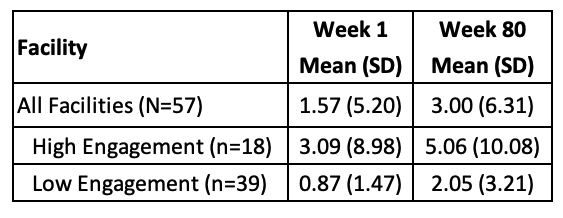 Table. Percentage of HLA-B*58:01 testing among allopurinol users with self-reported Asian and African American race, overall and by engagement group, at the beginning and the end of the study period.
Table. Percentage of HLA-B*58:01 testing among allopurinol users with self-reported Asian and African American race, overall and by engagement group, at the beginning and the end of the study period.
.jpg) Figure 1. Simulated gout dashboard displaying fictitious patient data for monitoring HLA-B*58:01 testing and serum urate levels. This example shows how users can filter and visualize patient-level data, including urate-lowering therapy use, recent lab results, and HLA-B*58:01 testing status.
Figure 1. Simulated gout dashboard displaying fictitious patient data for monitoring HLA-B*58:01 testing and serum urate levels. This example shows how users can filter and visualize patient-level data, including urate-lowering therapy use, recent lab results, and HLA-B*58:01 testing status.
.jpg) Figure 2. Interrupted time series (ITS) assessing HLA-B*58:01 testing pre- and post-dashboard launch
Figure 2. Interrupted time series (ITS) assessing HLA-B*58:01 testing pre- and post-dashboard launch
for high vs low-engagement facilities.
To cite this abstract in AMA style:
Becerril A, Li J, Wilson C, Tarasovsky G, Fadairo-Azinge A, Whooley M, Schmajuk g. National Implementation of a Medication Safety Dashboard to Improve HLA-B*58:01 Testing Among Allopurinol Users in the Veterans Health Administration [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/national-implementation-of-a-medication-safety-dashboard-to-improve-hla-b5801-testing-among-allopurinol-users-in-the-veterans-health-administration/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/national-implementation-of-a-medication-safety-dashboard-to-improve-hla-b5801-testing-among-allopurinol-users-in-the-veterans-health-administration/
