Session Information
Session Type: ACR Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: The Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial demonstrated that for adults with severe scleroderma (ACR 1995 criteria) and internal organ involvement, myeloablative CD34+selected autologous hematopoietic stem cell transplantation (HSCT) led to significantly improved clinical outcomes compared to 12 monthly infusions of cyclophosphamide (CYC)1. Participants were randomized between 2006 and 2011 and followed to 72 months. We report here survivor status, late effects and outcomes 6-11 years after randomization on the SCOT trial.
Methods: We conducted scripted telephone interviews with survivors and searched public death records in mid-2017. Endpoints included time to death or organ failure (see table). Current health status, physical functioning, toxicities, DMARD use and quality of life measures were solicited, and the current HAQ disability index and SF-36 health surveys compared to last assessments. Events are listed as occurring before or after prior reporting1.
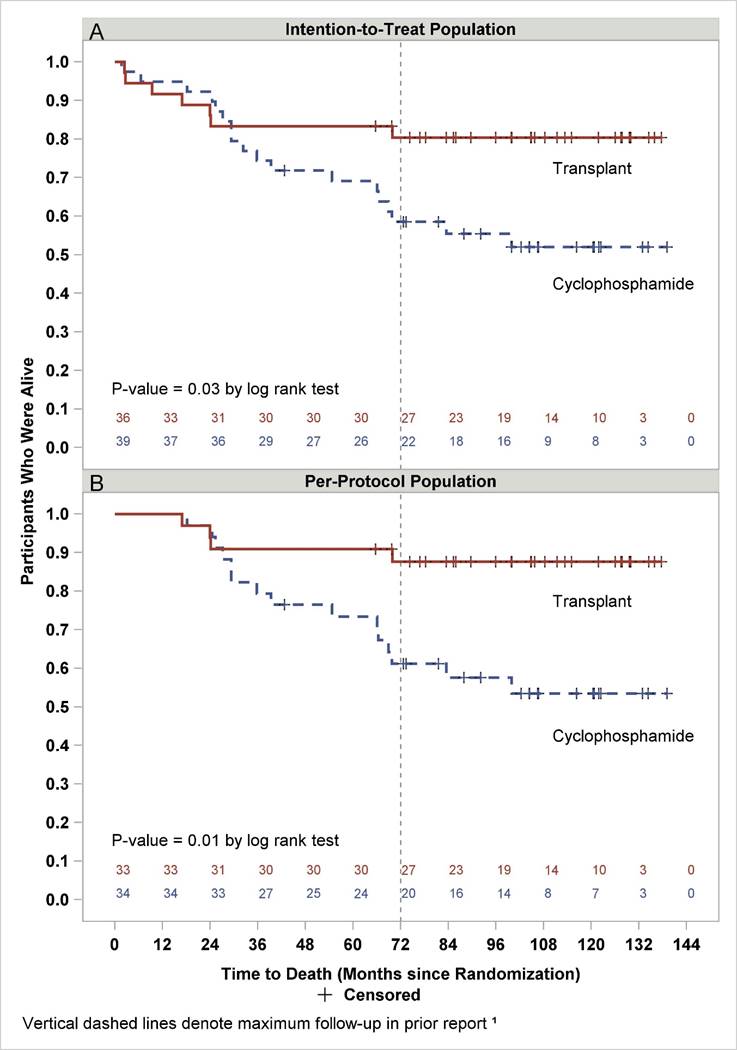
Results: Of 75 randomized SCOT participants, 7 HSCT and 18 CYC recipients have died. For HSCT, no new deaths were identified in the follow-up study compared to 4 new deaths for CYC (table A). Overall survival is depicted in the intention-to-treat (figure A) and per-protocol (received HSCT or >/= 9 doses CYC) (figure B) populations. At 11 years after randomization, Kaplan Meier estimates of overall survival were 80% vs 52% and 88% vs 53% (HSCT vs CYC; Figure A and B, p=0.03 and 0.01, respectively).
Among the 25 HSCT and 18 CYC follow-up participants, physical functioning and weight gain were improved following HSCT (table B). Organ failure developed in 2 HSCT (cardiac ablation and pacer) and 6 CYC recipients (3 heart failure, 2 oxygen use and lung transplant). No subsequent malignant or myelodysplastic events were observed. Performance status was significantly better after HSCT while employment rates and measures of physical and social functioning trended higher but were not statistically different. DMARDS were being taken by 2 HSCT and 7 CYC recipients, with 92% and 61% of the two respective treatment groups remaining DMARDs-free (p=0.01).
Conclusion: This follow-up analysis demonstrates that the clinical benefits of HSCT previously reported1 are durable 6-11 years after randomization. Survival and functional status were significantly better with HSCT, and continuing control of scleroderma was demonstrated by 92% of transplant survivors remaining free of DMARDs and disease.
Reference: 1Sullivan KM, et al. N Engl J Med 2018; 378:35-47.
To cite this abstract in AMA style:
Sullivan K, Pinckney A, Goldmuntz E, Welch B, Khanna D, Simms RW, Kafaja S, Georges G, Storek J, Csuka ME, Nash R, Furst DE, Crofford L, McSweeney P, Mayes MD, Keyes-Elstein L. Myeloablative Autologous Hematopoietic Stem Cell Transplantation for Severe Scleroderma: Long-Term Outcomes 6-11 Years after Entry on a Randomized Study Comparing Transplantation and Cyclophosphamide [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/myeloablative-autologous-hematopoietic-stem-cell-transplantation-for-severe-scleroderma-long-term-outcomes-6-11-years-after-entry-on-a-randomized-study-comparing-transplantation-and-cyclophosphamide/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/myeloablative-autologous-hematopoietic-stem-cell-transplantation-for-severe-scleroderma-long-term-outcomes-6-11-years-after-entry-on-a-randomized-study-comparing-transplantation-and-cyclophosphamide/