Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Mycophenolate mofetil (MMF) is a first-line immunosuppressant treatment for systemic sclerosis (SSc), particularly for patients with interstitial lung disease (ILD) and diffuse skin involvement. MMF persistence reflects its efficacy, the risk or occurrence of adverse events (AEs), the risk of relapse after discontinuation, and the availability of therapeutic alternatives. Real-life evidence about early and long-term adverse events (AEs) associated with the use of MMF in SSc is lacking, and predictors of drug discontinuation are not identified yet. We aimed to investigate a) the incidence and causes of MMF discontinuation due to AEs in SSc patients and b) the incidence and association of severe infections, lower airway infections, and unbearable gastrointestinal symptoms leading to MMF discontinuation.
Methods: Medical records of SSc patients treated with MMF from January 2012 to December 2021 and followed up in 9 tertiary centers were retrospectively collected and evaluated. Clinical and demographic data included AEs (i.e., infections, gastrointestinal (GI) intolerance, laboratory abnormalities, new cancer diagnosis) and reasons for dosage reduction or discontinuation. Infection severity was ranked according to the GREFIG study classification system. A competing risk analysis was performed to explore the association of AE-related MMF discontinuations with alternative causes of MMF discontinuation as a competing risk.
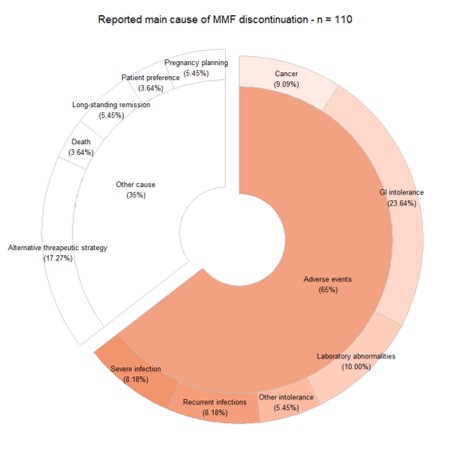
Results: Data from 545 SSc patients observed for 3.1 years (IQR 1.4-4.9) were analyzed. Combination therapy with steroids was recorded in 37.0% of patients, while in 14.7% of cases, MMF was associated with additional immunosuppressants. Almost 25% of patients did not tolerate the prescribed dose of MMF because of drug-related AEs. MMF discontinuation rate was 6.6/1000 patients-year (95% IC 5.3-8.0) with a 70.3% 5-year retention rate (95% IC 0.65-0.76). Gastrointestinal intolerance and infections (GREFIG grades 2-3) were the most common AEs leading to discontinuation with different time patterns. Infection severity tended to increase over time. Respiratory infections were the most commonly reported infections. The risk of major infections (grades 2-3) was associated with male gender (HR 1.9, 95% IC 1.3-2.9), anti-centromere antibody (HR 1.7, 1.1-2,6), pulmonary hypertension (HR 2.0, 95% IC 1.3-3.0), late capillaroscopy pattern (HR 1.5, 95% IC 1.0-2.3), and concomitant COPD (HR 3.0, 95% IC 1.4-6.4) at univariate analysis.
Conclusion: One in four SSc patients had to reduce or discontinue MMF due to adverse events, primarily gastrointestinal intolerance and infections. Factors linked with microvascular impairment served as a risk factor for severe infections during MMF treatment.
To cite this abstract in AMA style:
Bosello S, De Lorenzis e, verardi l, Natalello g, Cerasuolo p, Di Donato S, Pellegrino G, De Luca G, Campochiaro C, Lepri G, Cometi L, Cacciapaglia F, Armetano G, De Pinto M, Motta F, De Santis M, Giuggioli D, Del Papa N, Iannone F, Guiducci S, Riccieri v, D'Agostino M, Del Galdo F. Mycophenolate Mofetil Use in Clinical Practice: Persistence on Therapy and Long-term Adverse Events in a Multicentric Cohort of Scleroderma Patients [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/mycophenolate-mofetil-use-in-clinical-practice-persistence-on-therapy-and-long-term-adverse-events-in-a-multicentric-cohort-of-scleroderma-patients/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mycophenolate-mofetil-use-in-clinical-practice-persistence-on-therapy-and-long-term-adverse-events-in-a-multicentric-cohort-of-scleroderma-patients/