Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Interstitial lung disease (ILD) is the leading cause of morbidity and mortality in systemic sclerosis (SSc). The majority of studies investigating novel therapies for SSc-ILD use the forced vital capacity (FVC) as the primary outcome. However, changes in the FVC may not consistently translate into clinically meaningful improvements from a patient’s perspective. This study evaluated whether treatment with cyclophosphamide (CYC) and mycophenolate (MMF) improves health-related quality of life (HRQOL) among SSc patients with active ILD.
Methods: This study examined outcomes in patients (N=142) who participated in Scleroderma Lung Study (SLS) II (Tashkin et al. Lancet Resp Med 2016), a randomized controlled trial comparing mycophenolate (MMF) for 2 years versus oral CYC for 1 year followed by 1 year of placebo in patients with relatively early SSc-ILD. The following HRQOL outcomes were examined: Short Form 36 (SF-36), Health Assessment Questionnaire (HAQ) disability index (DI), Baseline and Transitional Dyspnea Index (BDI, TDI), the Leicester Cough Questionnaire (LCQ), St. George’s Respiratory Questionnaire (SGRQ), and the Scleroderma Clinical Trials Consortium Gastrointestinal Tract (GIT) 2.0. The differences in HRQOL scores from baseline to 24 months were measured in each treatment arm. The proportion of subjects in each treatment group whose scores improved at >= the minimum clinically important difference (MCID) in HRQOL measures was assessed. Correlations between changes in HRQOL and those in FVC%-predicted were also examined using Pearson correlation coefficient.
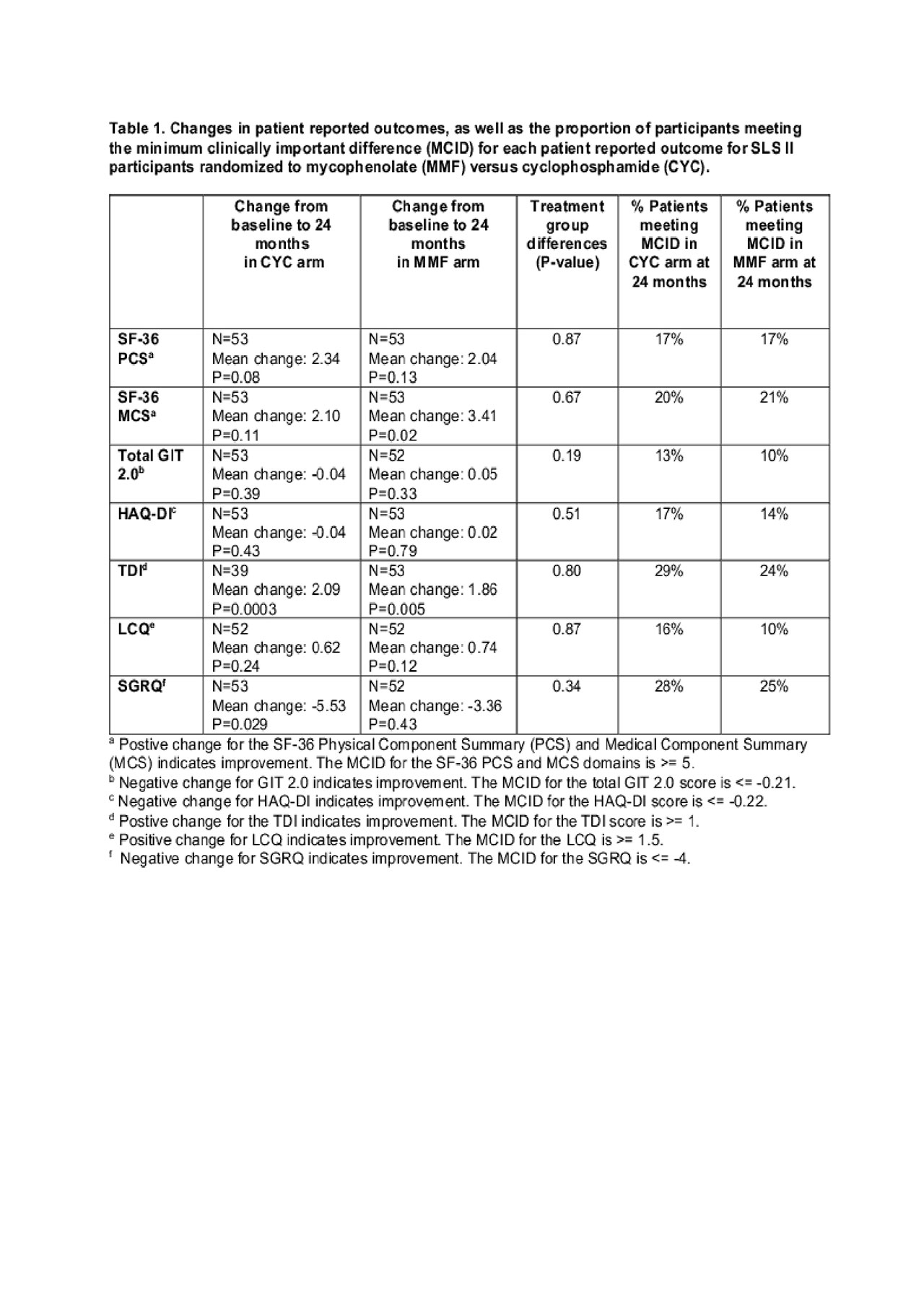
Results: Treatment with CYC and MMF led to improvements in HRQOL outcomes, with no appreciable between treatment arm differences (Table 1). The TDI improved significantly in both treatment arms (CYC: P=0.0003; MMF: P=0.005), and 29% and 24% of CYC and MMF patients, respectively, met or exceeded MCID estimates. For the HAQ-DI, 17% and 14% of CYC and MMF patients, respectively, met or exceeded the MCID estimates. For Total SGRQ scores 28% and 25% of CYC and MMF patients, respectively, met or exceeded MCID estimates. At baseline, the FVC%-predicted did not correlate with any of the HRQOL outcomes (coefficients with HRQOL ranged from 0.01 to 0.11; data not shown) The 24-month change in the FVC-% predicted correlated weakly with only a few of the changes in HRQOL scores (HAQ-DI r=-0.30; TDI r=-0.40; SF-36 Physical Component Summary r=0.30).
Conclusion: Treatment with CYC and MMF improved overall HRQOL in patients with SSc-ILD, including a large proportion who improved in dyspnea and respiratory HRQOL by >= MCID estimates. The relationship between HRQOL measures and changes in the FVC was relatively weak, suggesting that changes in the PROs provide additional information about treatment efficacy not captured by changes in the FVC alone in this patient population. The overall improvement in FVC%, along with HRQOL in the SLS-II, supports the treatment of SSc-ILD with immunosuppressive therapies. Future SSc-ILD trials should include PROs as they directly measure how a patient feels and functions.
To cite this abstract in AMA style:
Volkmann E, Tashkin D, Roth M, LeClair H, Clements P, Furst D, Elashoff R, Khanna D. Mycophenolate Mofetil and Cyclophosphamide Improve Health-Related Quality of Life in Patients with Systemic Sclerosis Who Participated in SLS II [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/mycophenolate-mofetil-and-cyclophosphamide-improve-health-related-quality-of-life-in-patients-with-systemic-sclerosis-who-participated-in-sls-ii/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mycophenolate-mofetil-and-cyclophosphamide-improve-health-related-quality-of-life-in-patients-with-systemic-sclerosis-who-participated-in-sls-ii/