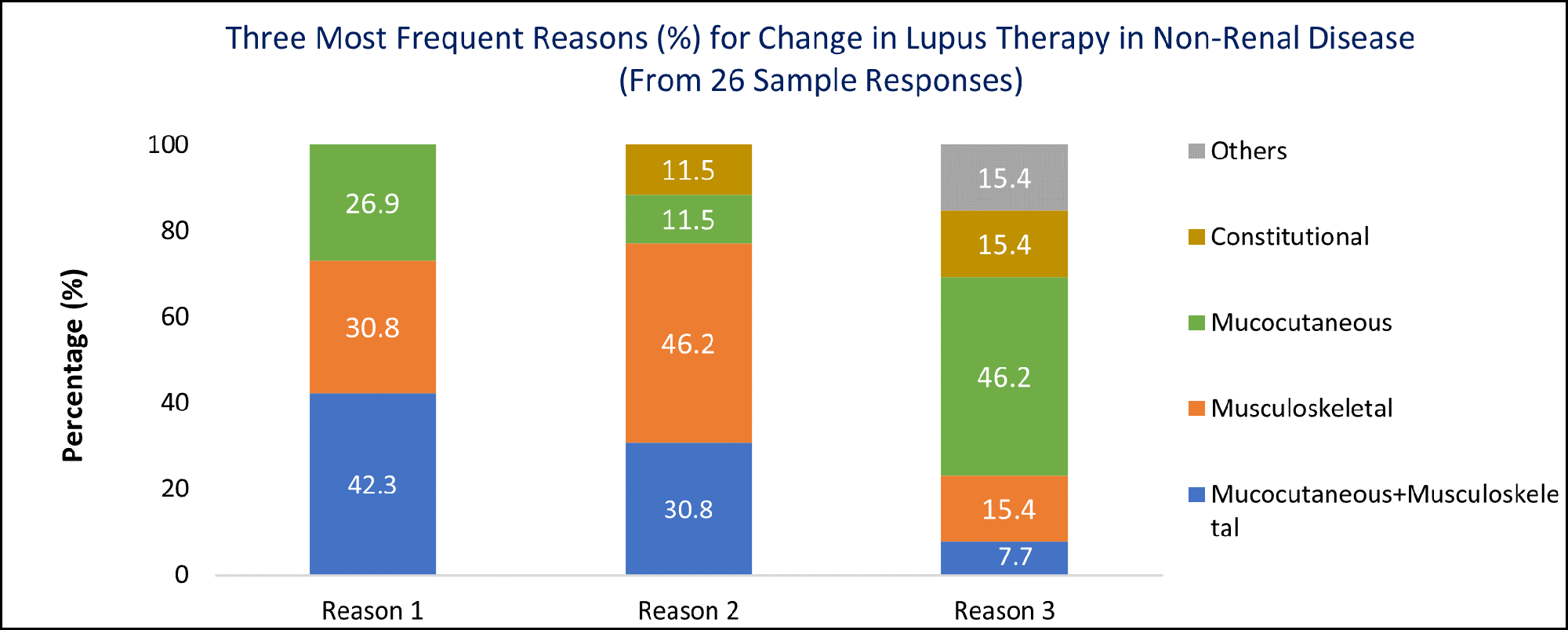Session Information
Date: Monday, November 13, 2023
Title: (1442–1487) SLE – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Musculoskeletal manifestations of systemic lupus erythematosus (SLE) are common, consisting ofarthralgia and arthritis (95%), myalgia/myositis (17%), tenosynovitis, and bursitis (12%). Joint involvement is a common reason for inclusion in SLE clinical trials. Involvement of ≥2 joints with pain and signs of inflammation satisfies 4 points required to meet trial entry criteria [SLE Disease Activity Index 2000 (SLEDAI-2K) ≥6 points and “Clinical” SLEDAI-2K ≥4 points). Likewise, the SRI-4 response which includes ≥4-point reduction in SLEDAI-2K score can be achieved through joint improvement alone and may allow patients with lower ‘initial’ joint counts to meet the response threshold of involvement of < 2 joints at the trial end with greater ease compared to those with higher entry joint counts. Additionally, not all joint symptoms in SLE patients are directly attributable to lupus, as comorbid osteonecrosis, osteoarthritis, and fibromyalgia can contribute to joint pain, and may not respond to SLE-directed therapy, thus influencing end-outcome attainment. To gain further perspectives into SLE joint involvement, a survey was conducted among lupus experts within the Lupus Clinical Investigators Network (LuCIN) network, a North American consortium of investigators from 54 academic sites who are actively involved in clinical trials.
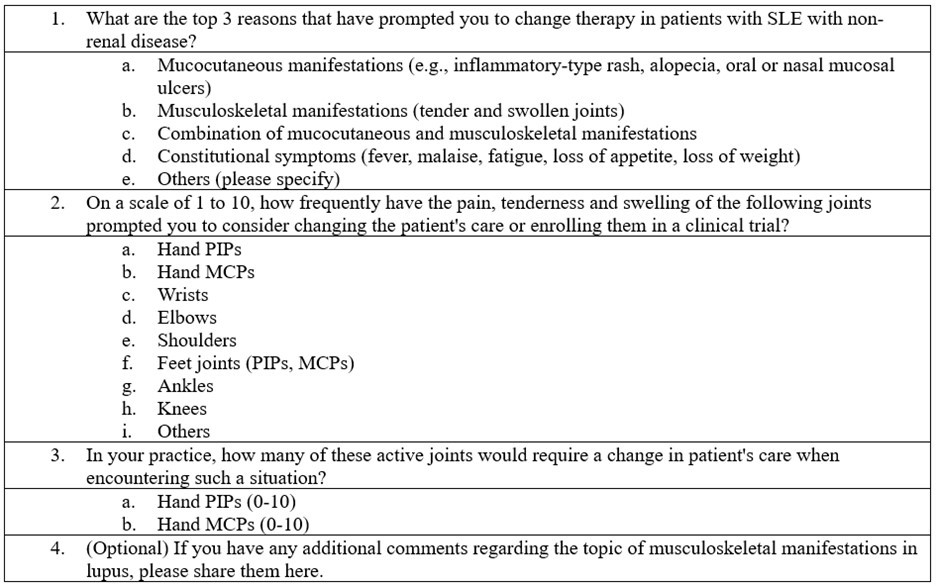
Methods: The survey was created in Google Forms with questions focused on the most frequent reasons for therapy change in lupus, the frequency of specific joint involvements that prompted therapy change and enrollment into clinical trials, and the number of active joints necessitating a change in patient care (Table 1). The survey was disseminated via email to LuCIN investigators.
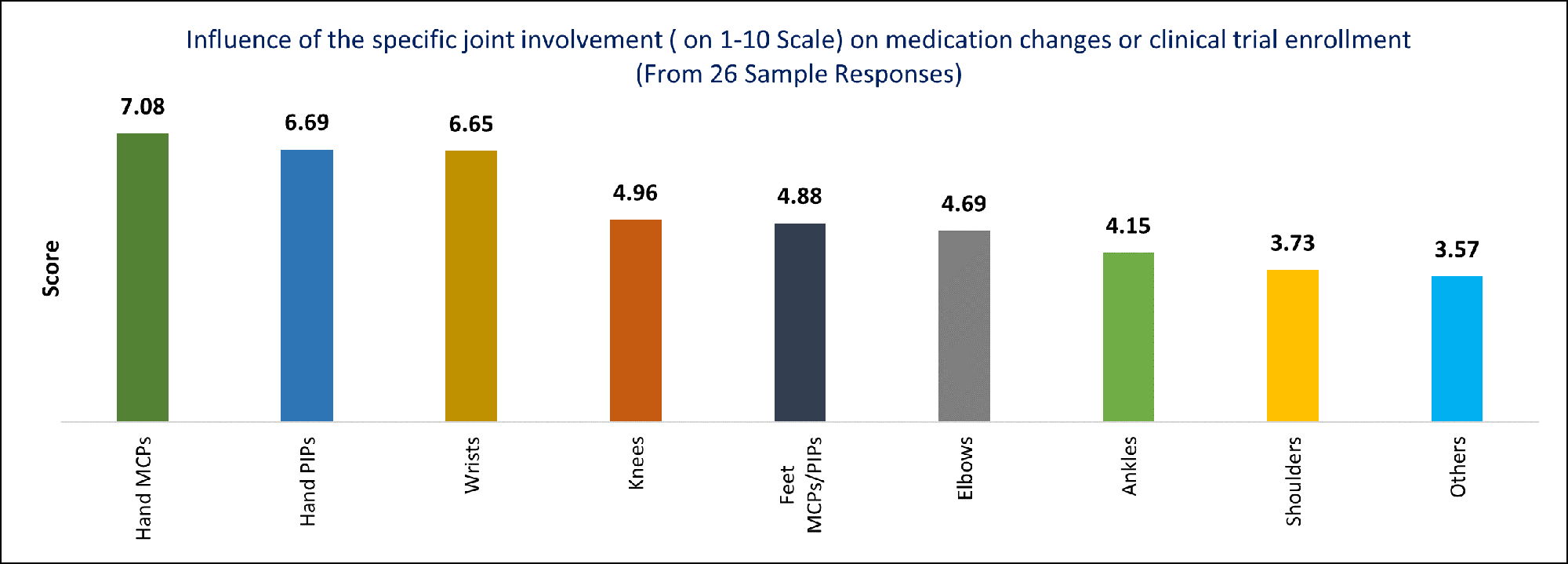
Results: Out of 54 LuCIN members, 26 responded to the survey. Joint involvement was identified as the most frequent reason for therapy change in non-renal SLE by 30.8% of respondents, and the 2nd and 3rd top reasons by 46.2% and 15.4% of respondents (Figure 1). The combination of joint and mucocutaneous manifestations was cited as the top reason for therapy change by 42.3% (11/26). MCPs, hand PIPs, wrists, and knees were the joints most frequently associated with therapy change or enrollment in clinical trials (Figure 2). The respondents indicated that approximately 3- 4 active MCPs or hand PIPs would prompt a change in therapy.
Conclusion: Joints and the combination of joint and mucocutaneous manifestations were identified as the most common reasons for therapy change in non-renal SLE. MCPs, hand PIPs, wrists, and knees were identified as joints most frequently associated with the decision to change therapy or enroll in a clinical trial. Future Directions: Understanding the pattern and extent of joint involvement over time using data from completed prospective clinical trials may contribute to a more comprehensive understanding of SLE joint manifestations and their influence on trial outcomes. Refined clinical assessment of joints using advanced imaging techniques may improve the characterization of joints and the assessment of interventions in both clinical practice and clinical trials.
To cite this abstract in AMA style:
Dhital R, Dall'Era M, Kalunian K. Musculoskeletal Involvement in Systemic Lupus Erythematosus: Insights from a Survey of Lupus Experts [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/musculoskeletal-involvement-in-systemic-lupus-erythematosus-insights-from-a-survey-of-lupus-experts/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/musculoskeletal-involvement-in-systemic-lupus-erythematosus-insights-from-a-survey-of-lupus-experts/