Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Immune checkpoint inhibitors (ICI) belong to a novel group of biologic agents used to treat cancer. ICI increase the immune response to tumor cells by blocking cancer induced immune inhibitory pathways. Immune-related adverse effects (irAE) are a recognized complication of these drugs. Musculoskeletal (MSK) irAE have been described. We studied irAE of ICI in Hispanics at a single site in Puerto Rico, their management and outcome. We report the MSK irAE identified in the population studied.
Methods: A retrospective medical record analysis of 193 patients treated with an ICI was conducted at the Cancer Center of Hospital Auxilio Mutuo in Puerto Rico, beginning from the first therapy with ICI to August 31, 2020. Data abstracted included demographic information, type of cancer, ICI used alone, in combination or sequentially, dose and cycles of ICI, treatment prescribed prior to and during ICI use, comorbidities, development and type of irAE, time to development of irAE from start of ICI, management and outcome of irAE, and autoimmune diseases diagnosed prior to ICI use.
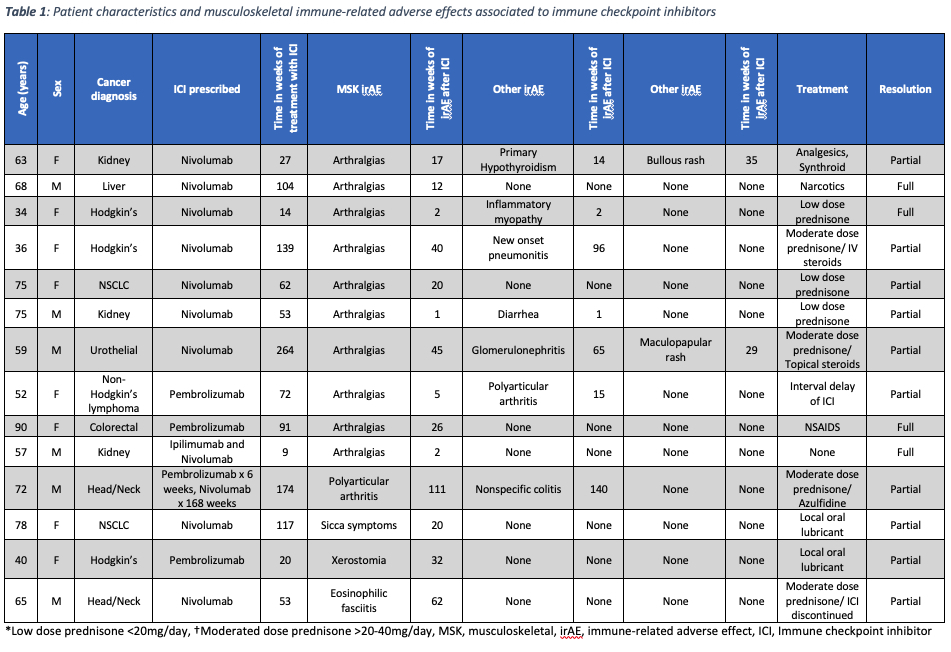
Results: A total of 16 MSK irAE were identified among 14 (7.25%) patients. MSK adverse effects were the third most common irAE identified (8%), following skin (17%) and gastrointestinal involvement (9%). Nine patients were female, and 5 patients were male with a mean age of 63 years (range 34-90).
MSK irAE were observed in 9 of 97 (9.3%) patients that were treated with nivolumab monotherapy and in 3 of 75 (4%) treated with pembrolizumab monotherapy. One in five (20%) patients treated with pembrolizumab followed by nivolumab had a MSK irAE and 1 of 20 (5%) treated with combination ipilimumab and nivolumab experienced a MSK irAE. The most common MSK irAE was arthralgia in 10 patients (71%), polyarticular arthritis in 2 patients (14%), followed by eosinophilic fasciitis, inflammatory myopathy, sicca syndrome, and xerostomia in 7% respectively. The mean time from starting ICI therapy to the development of a MSK irAE was 17 weeks (range 1-111).
Management of the irAE included prescription of corticosteroids, NSAIDs, sulfasalazine and delay or discontinuation of ICI therapy. Full resolution of the MSK irAE was achieved in 4 patients, while partial resolution was achieved in 10.
Conclusion: ICI MSK irAE were observed in 7.25% of the Hispanic population studied. The most frequent MSK irAE was arthralgias (71%), which was associated with the use of nivolumab as monotherapy in 7 of 10 patients. Two patients (12.5%) experienced more than 1 MSK irAE. However, 5 of 14 patients (35.7%) had an MSK irAE that concurred with a non-MSK irAE. Treatment of the irAE achieved full resolution in 29% of patients. ICI discontinuation or delay in prescription was required in 2 patients and 1 patient required hospitalization. No patients with a prior MSK autoimmune disease had a relapse of their disease on ICI therapy.
To cite this abstract in AMA style:
Santiago A, Vila S, Cabanillas F. Musculoskeletal Immune-Related Adverse Effects of Immune Checkpoint Inhibitors at a Single Site in a Hispanic Population in Puerto Rico [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/musculoskeletal-immune-related-adverse-effects-of-immune-checkpoint-inhibitors-at-a-single-site-in-a-hispanic-population-in-puerto-rico/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/musculoskeletal-immune-related-adverse-effects-of-immune-checkpoint-inhibitors-at-a-single-site-in-a-hispanic-population-in-puerto-rico/