Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Advances in treatment for RA may have resulted in a decrease in musculoskeletal hospital admissions. This study aimed to assess the trends in hospital admissions among patients receiving treatment for RA in the past decade compared with the general population.
Methods: Patients with RA in the PHARMO Database Network treated with any disease-modifying antirheumatic drug (DMARD) between 1999 and 2010 were identified. Population controls were randomly matched 2:1 to patients with RA by age and sex. Patients and controls were followed from the first DMARD dispensed and the matched index date, respectively, until death, end of study period or end of database registration, whichever occurred first. Musculoskeletal hospital admissions and procedures were assessed during follow-up. Incidence rates (IRs) of the first musculoskeletal hospitalization after the index date were calculated and compared using Cox proportional hazards regression.
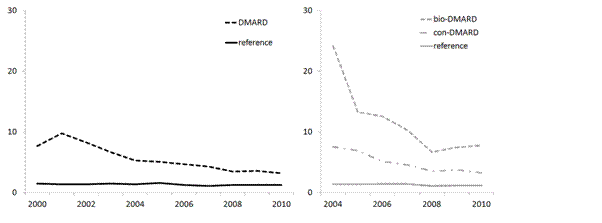
Results: Between 1999 and 2010, 24,762 patients with RA treated with DMARDs were identified. The IR of musculoskeletal admissions per 100 person years (pt-yrs) decreased from 10.4 (95% CI] 9.4, 11.5) in 1999 to 3.2 (95% CI 2.9, 3.5) in 2010, while the reference population IR was stable at 1.3 per 100 pt-yrs. The largest improvement among patients with RA occurred around 2003; the period in which biologic DMARDs (bDMARDs) were introduced. Therefore, a second comparison was made between 2078 patients with RA (8%) treated with bDMARDs (index date=first bDMARD dispensed) and 4156 patients with RA (17%) treated only with conventional DMARDs, matched by age, sex and timing of first DMARD dispensed (any) in the database. Between 2003 and 2010, the IR of musculoskeletal hospital admissions per 100 pt-yrs decreased from 27.1 (95% CI 20.4, 35.2) to 7.8 (95% CI 6.0, 9.9) among bDMARD users and from 9.9 (95% CI 7.4, 12.9) to 3.2 (95% CI 2.6, 4.1) among the matched conventional DMARD users. Also, the IR of musculoskeletal procedures per 100 pt-yrs decreased from 4.3 (95% CI 2.7, 6.3) to 1.8 (95% CI 1.3, 2.4) among bDMARD users and from 1.6 (95% CI 0.7, 2.9) to 0.7 (95% CI 0.4, 1.1) among the matched conventional DMARD users. Compared with the general population, bDMARD users in 2010 remained six times more likely to be hospitalized for a musculoskeletal event (hazard ratio 6.5 [95% CI 4.2, 10.0]).
Conclusion: The IR of musculoskeletal hospital admissions among patients in the Netherlands treated for RA decreased from 1999 to 2010. The largest reduction occurred during the period when bDMARDs were introduced. However, the rates have stabilized in recent years and hospital admission rates in 2010 remained twice as high for patients with RA as the general population, and six times higher in patients receiving bDMARDs.
Figure: IRs per 100 pt-yrs of musculoskeletal hospital admissions among DMARD users and matched references.
Disclosure:
I. D. Bezemer,
PHARMO Institute for Drug Outcomes Research,
9;
L. M. A. Houweling,
PHARMO Institute for Drug Outcomes Research,
9;
E. Alemao,
Bristol-Myers Squibb,
1,
Bristol-Myers Squibb,
3;
F. J. A. Penning-van Beest,
PHARMO Institute for Drug Outcomes Research,
9;
M. Hochberg,
Abbott Laboratories, Amgen Inc., BMS, Eli Lilly and Company, EMD Serono Inc., Genentech/Roche, Merck & Co., Inc., Novartis Pharma AG, Pfizer Inc,
5,
Bioberica SA, IBSA,
8,
NIH,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/musculoskeletal-hospital-admissions-among-patients-treated-for-rheumatoid-arthritis-between-1999-and-2010-compared-with-the-general-population-in-the-netherlands/