Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: RA – Treatment I: Switching or Discontinuation of Therapies
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: PF-06410293 (ADL-afzb) is an adalimumab (ADL) biosimilar approved in the US, the EU, and 19 other countries. Biosimilarity of ADL-afzb with reference ADL (ADL-REF) was confirmed in a comparative, randomized, double-blind, parallel group Phase 3 clinical trial in RA.1,2 For submission to US Food and Drug Administration for biosimilar interchangeability approval,3 this open-label, two-arm, randomized, parallel group study assessed the impact of switching between ADL-REF and ADL-afzb compared with continuous dosing with ADL-REF, on steady-state serum ADL pharmacokinetics (PK) in RA. Safety and immunogenicity were evaluated
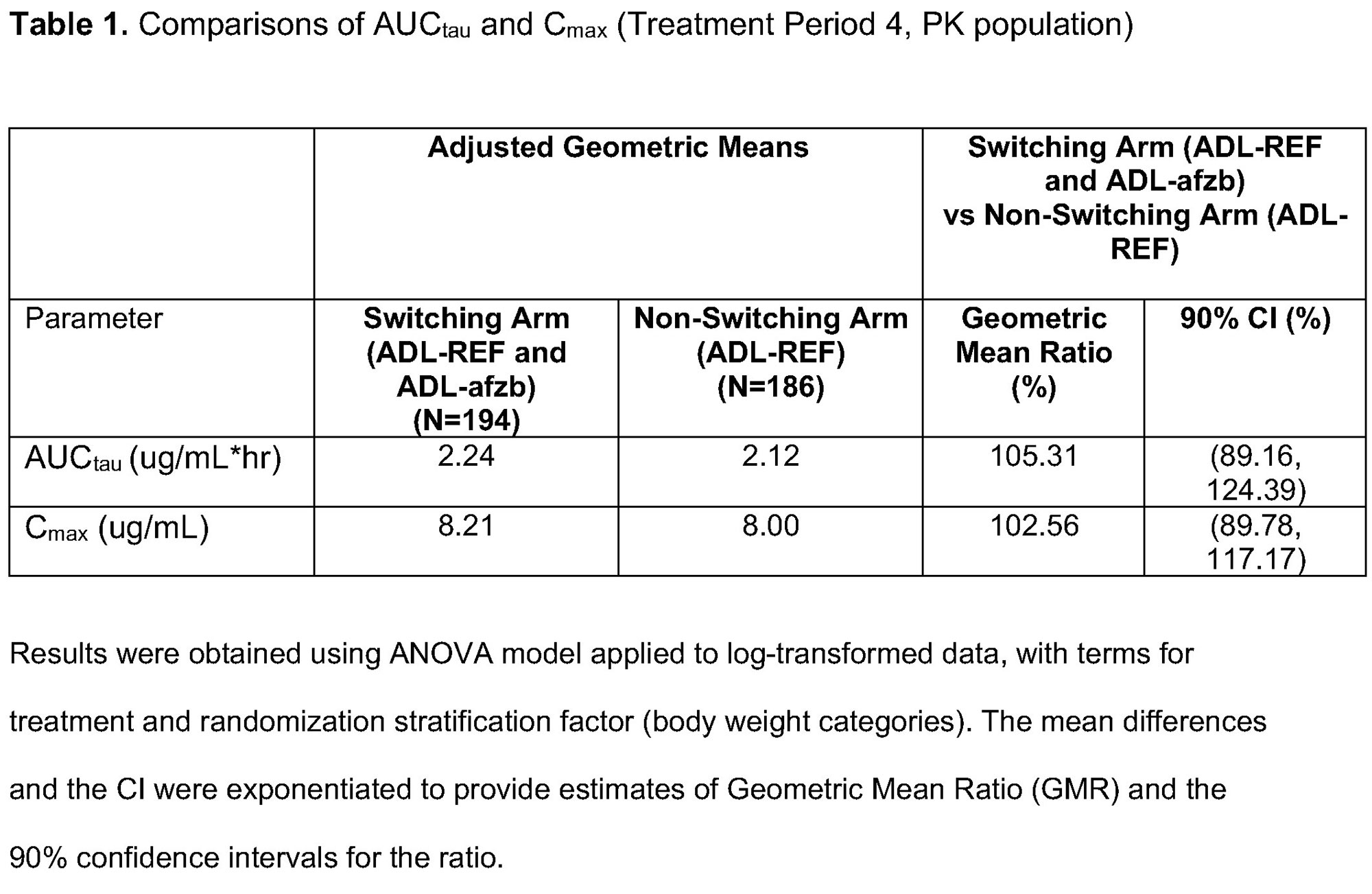
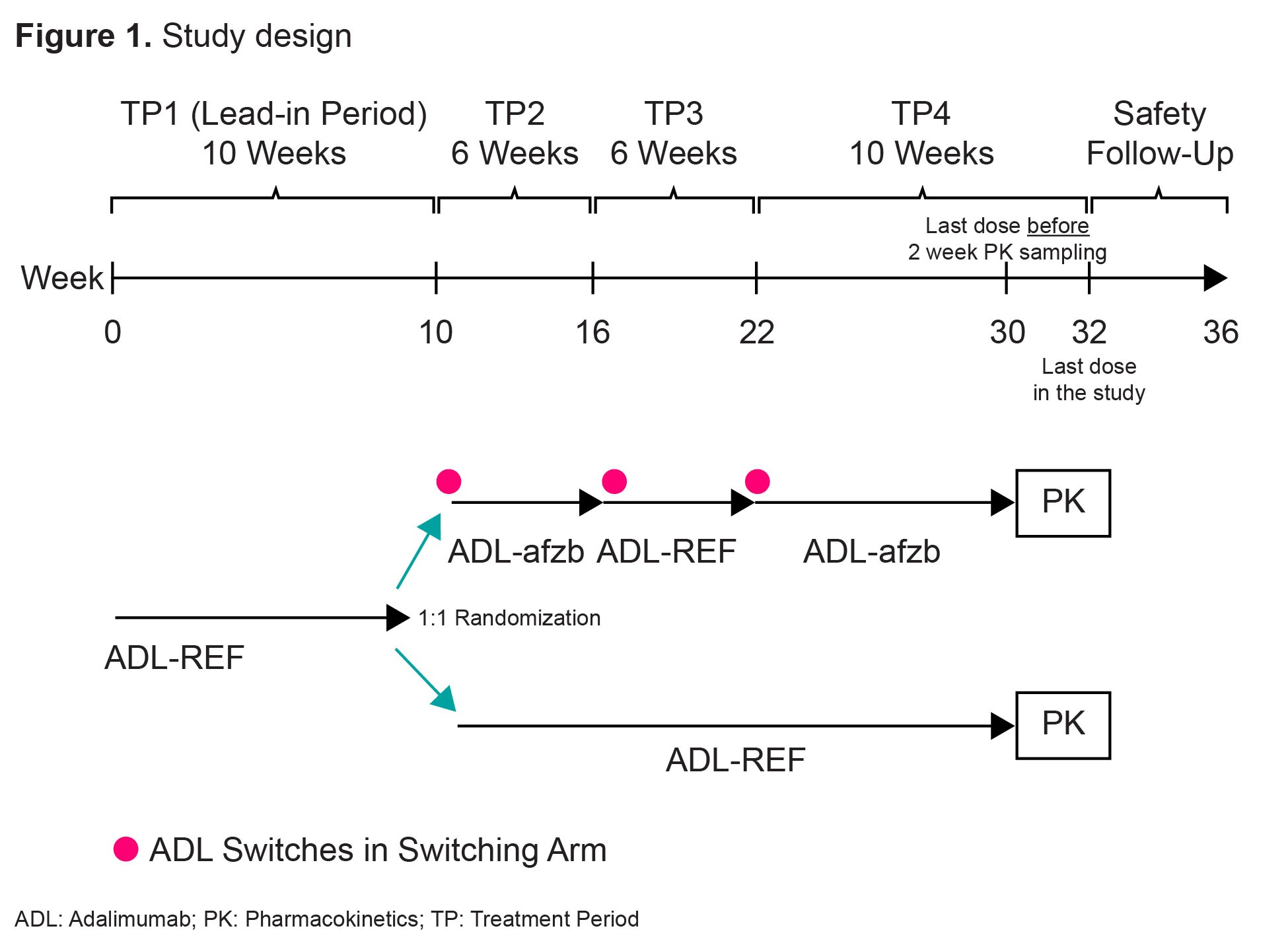
Methods: Patients with active RA receiving methotrexate (MTX) for ≥12 weeks were randomized to one of two treatment groups. The switching arm included switches between ADL-REF and ADL-afzb. The non-switching arm included continuous ADL-REF. There were 4 treatment periods (TPs) and 3 switches in the switching arm (ADL-REF during TP1 and TP3, ADL-afzb during TP2 and TP4) with a Safety Follow-Up period after TP4 (Fig 1). Patients received 40mg of ADL-afzb or ADL-REF plus MTX throughout. Primary endpoints maximum observed serum concentration (Cmax) and area under plasma concentration-time curve over dosing interval (AUCtau) were obtained during a 2-week PK intense sampling interval (Week 30 pre-dose-Week 32 pre-dose) where ADL serum concentrations were measured. Secondary PK parameters Tmax, Cav, and CL/F were obtained during the same interval. Immunogenicity and safety endpoints included % patients with ADA/Nab (and their titers) over time and adverse events (AEs).
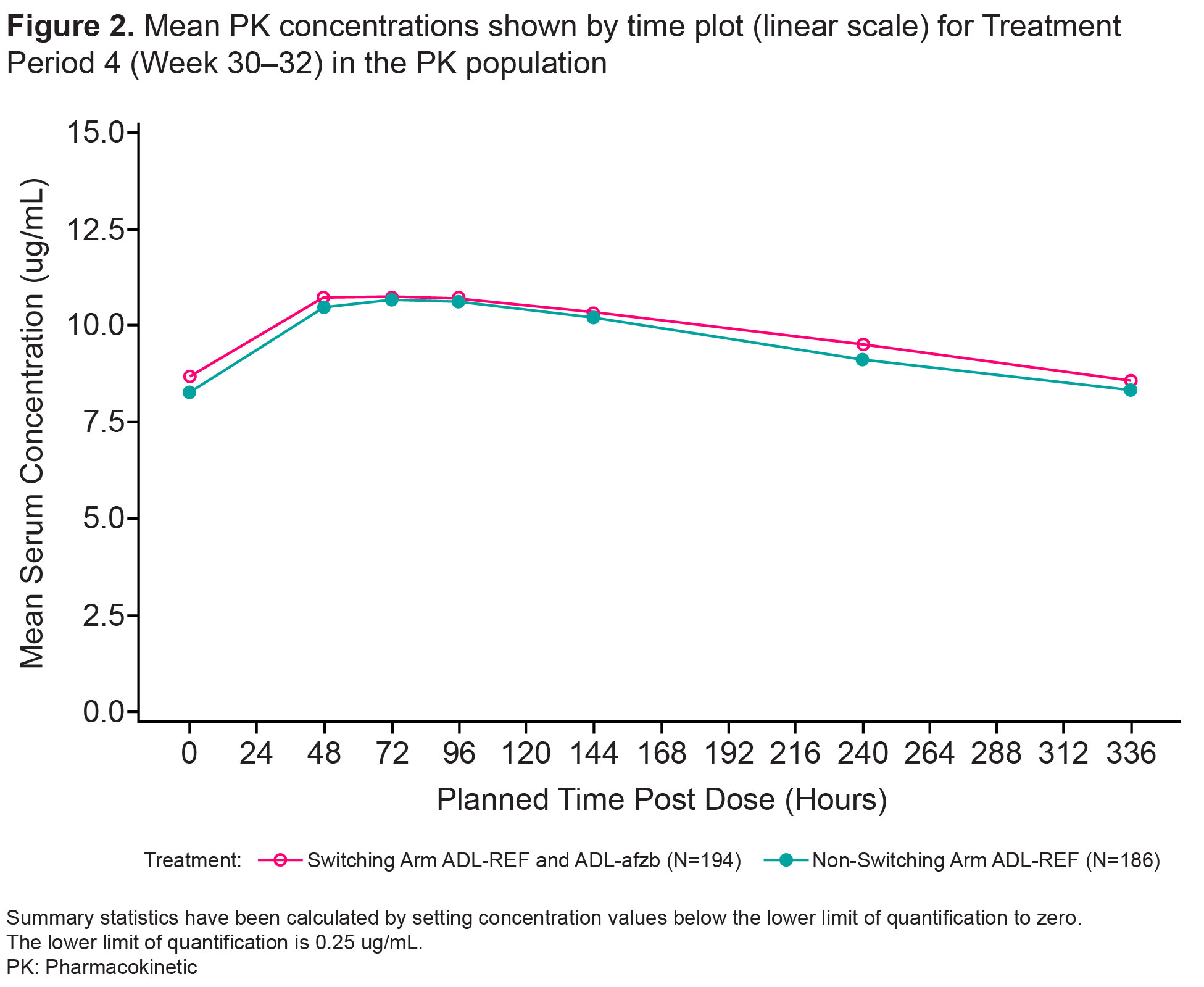
Results: Patient disposition was balanced. TP1: 445 patients enrolled, 427 completed,18 (4%) patients discontinued (12/18 due to AE). TP2 and beyond: of 213 patients in the switching arm, 12 (6%) patients discontinued (9/12 due to AE); of 214 patients in the non-switching arm, 20 (9%) discontinued (13/20 due to AE). Primary endpoint results (Cmax, AUCtau) at Week 30-32 demonstrated PK equivalence between switching and non-switching arms. 90% CIs for geometric mean ratios of Cmax and AUCtau were within the pre-specified equivalence margin of 80-125% (Table 1). Secondary endpoints Tmax, Cav, and CL/F were similar in both arms as were mean PK concentration profiles (Fig 2). The % of ADA/NAb positive patients was similar at Week 10 (randomization) and remained similar between switching and non-switching arms throughout the study as were ADA/NAb titers by visit. The % of patients with potentially immunogenic reactions to drug by ADA status over time was low, with no clinically significant differences between the two arms. There were no clinically meaningful differences in safety outcomes between the two arms, as represented by SAEs, AESIs, Grade 3 or higher AEs/AESIs, and no deaths.
Conclusion: Results support that the risk of switching between ADL-REF and ADL-afzb in terms of immunogenicity, safety, or possible diminished efficacy (using PK as surrogate) is not greater than the risk of using ADL-REF without the switch.
1.Fleischmann RM, et al. Arthritis Res Ther. 2018;20:178
2.Fleischmann R, et al. Ann Rheum Dis. 2020;79:1439-40
3.US FDA. https://www.fda.gov/media/124907/download
To cite this abstract in AMA style:
Fleischmann R, Lakhanpal S, Saikali W, Alvarez D, Cox D, Ianos C, Wang K, Zhang W. Multiple Switching Between the Biosimilar Adalimumab PF-06410293 (Abrilada™) and Reference Adalimumab (Humira®)in Combination with Methotrexate in Patients with Moderately to Severely Active Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/multiple-switching-between-the-biosimilar-adalimumab-pf-06410293-abrilada-and-reference-adalimumab-humirain-combination-with-methotrexate-in-patients-with-moderately-to-severely-act/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multiple-switching-between-the-biosimilar-adalimumab-pf-06410293-abrilada-and-reference-adalimumab-humirain-combination-with-methotrexate-in-patients-with-moderately-to-severely-act/