Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Upadacitinib (UPA) is a Janus Kinase inhibitor (JAKi) selective for JAK1, approved for the treatment of both Psoriatic Arthritis (PsA) and Axial Spondyloarthritis (axSpA). In May 2022, the Basque Health Service prioritized UPA use over other JAK inhibitors following the failure of Adalimumab biosimilars. The aim of this study is to describe the demographic and clinical characteristics of patients with axSpA and PsA treated with UPA and to explore sex-related differences.
Methods: 7 hospitals in our region (1.5 million inhabitants) participated in a multicenter, observational and ambispective study that included patients with UPA prescription from June 2021 to May 2024, with follow-up until Dec 2024. Among the 415 patients enrolled, 87 were diagnosed with AxSpA and 78 with PsA. Data were collected on comorbidities, disease duration and pattern, extra-articular manifestations (EAMs) and disease activity (measured by BASDAI and ASDAS for AxSpA, and DAPSA for PsA)
Results: Axial Spondyloarthritis (AxSpA) Subgroup (n=87): 49.1% were women, with a mean age of 52.2 years (SD 11.6) and a mean disease duration of 102.2 months (SD 92.7), longer in men (123.7 vs. 76.3 months). HLA-B27 was positive in 55%, 65.1% had radiographic disease, and 19.3% had ankylosis (more frequent in men). Peripheral manifestations were observed in 51.2% (arthritis), 23.5% (enthesitis). EAMs included inflammatory bowel disease (16.5%), uveitis (9.6%), and psoriasis (9.9%). UPA median persistence was 13.1 months (SE 2.73), with no significant difference by sex or prior biologic exposure. The disease activity was shown in Figure 1.Psoriatic Arthritis (PsA) Subgroup (n=78): 54.5% were women, with a mean age of 53.5 years (SD 10.2) and a disease duration of 116.5 months (SD 93.9). Age at diagnosis was later in women (46.6 vs. 40.3 years). The majority had peripheral (61.5%) or mixed (35.9%) patterns, with axial-only forms being rare (2 men). Women more frequently presented with peripheral PsA (76%), while men showed more mixed forms (51.4%). Cutaneous psoriasis was present in 80.8%, mostly plaque-type (65%), with no sex-based differences.The disease activity was shown in Figure 2. UPA median persistence was 19 months (95% CI 11.6), slightly longer in women (16.4 months in men), though not statistically significant. Sex-related differences in clinical presentation were observed in both diseases: men had longer disease duration and more axial involvement, while women had more peripheral manifestations and delayed diagnosis. Despite these differences, there were no significant sex-based differences in treatment history, response to UPA, or persistence in either AxSpA or PsA. A non-significant trend toward better persistence in women with PsA was noted.
Conclusion: UPA appears effective in the treatment of both AxSpA and PsA, even among patients with refractory and long-standing disease. While sex-based differences in clinical patterns were observed, outcomes and persistence were similar between men and women. These findings support the role of UPA across the Spondyloarthritis spectrum. Further studies with larger sample sizes are warranted to confirm potential sex-related differences in treatment outcomes.
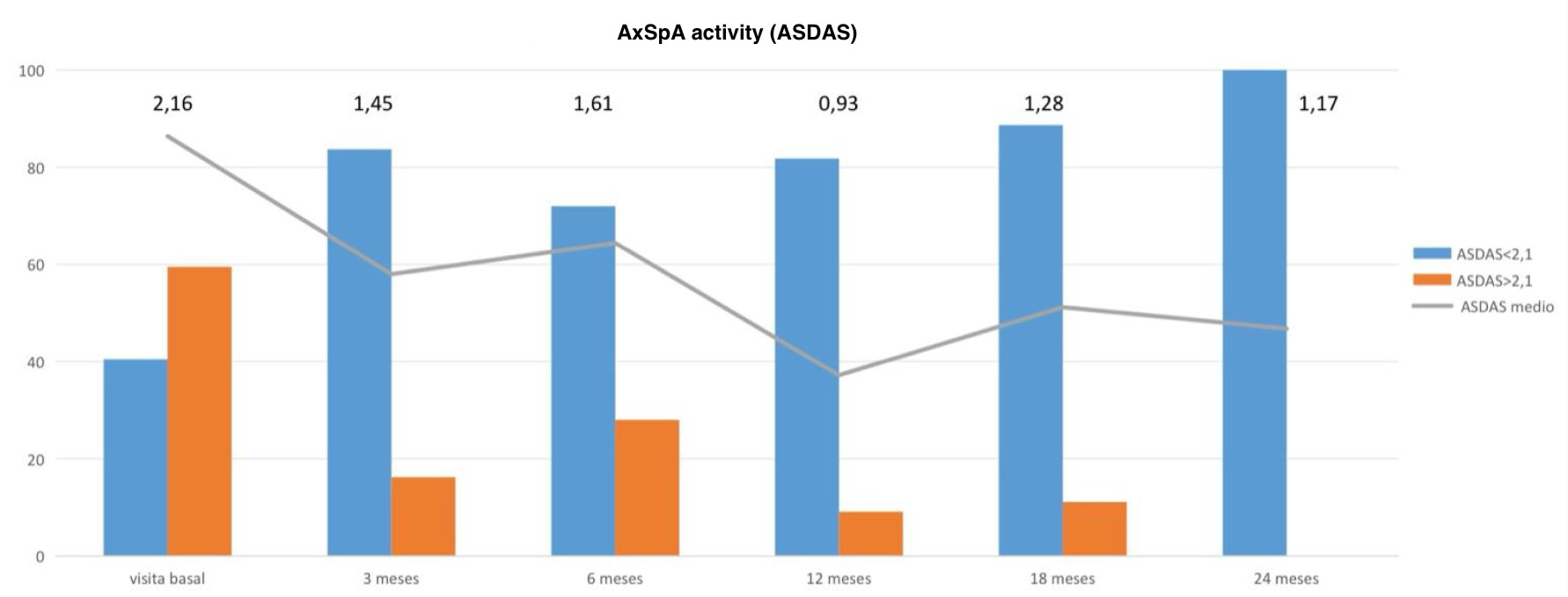 FIgure 1. AxSpA Activity (ASDAS).
FIgure 1. AxSpA Activity (ASDAS).
.jpg) Figure 2. ApS activity (DAPSA)
Figure 2. ApS activity (DAPSA)
To cite this abstract in AMA style:
Gonzalez Mozo de Rosales G, Lopez-Dominguez L, Alcorta-Lorenzo N, ibarguengoitia Barrena o, Montero D, Ruibal Escribano A, barastay Alberdi e, Valero Jaimes J, Ibarrola L, Garcia Escudero P, Lopez I Gomez M, Galindez Aguirregoicoa E, Garcia Vivar M. Multicenter study on the use of Upadacitinib: sex specific results in Spondyloarthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/multicenter-study-on-the-use-of-upadacitinib-sex-specific-results-in-spondyloarthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multicenter-study-on-the-use-of-upadacitinib-sex-specific-results-in-spondyloarthritis/
