Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The soluble guanylate cyclase stimulator riociguat (RIO) is a vasodilator with efficacy in patients with pulmonary arterial hypertension associated with connective tissue disease. Our objective was to evaluate the efficacy and safety of RIO in patients with systemic sclerosis-associated digital ulcers (SSc-DU) (NCT02915835).
Methods: Eligible subjects (with active or painful indeterminate DUs) were randomized in a 1:1 ratio to either placebo (PLA, n = 8) or RIO (n = 9) in individualized doses (maximum of 2.5 mg three times daily) during an 8-week titration period, followed by an 8-week stable dosing period.PDE5 inhibitors were not allowed. This was followed by an optional 16-week open-label extension phase for participants with active DUs / reoccurrence of DUs within 1 month of the end of the main treatment phase. The primary endpoint was the change from baseline to week 16 in net ulcer burden (NUB), analyzed using ANCOVA. Other endpoints included plasma biomarkers and the proportion of participants with treatment-emergent adverse events (AEs).
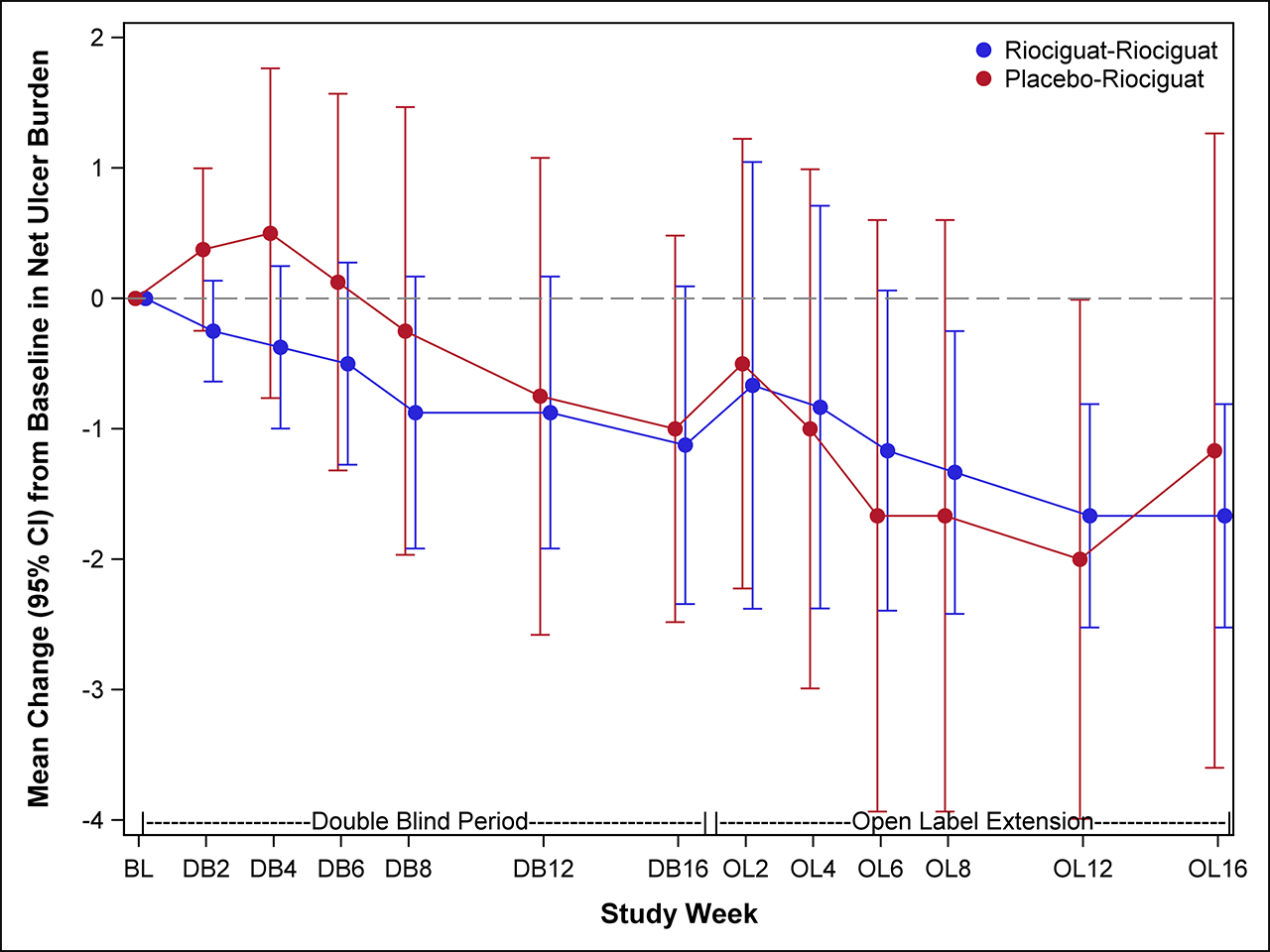
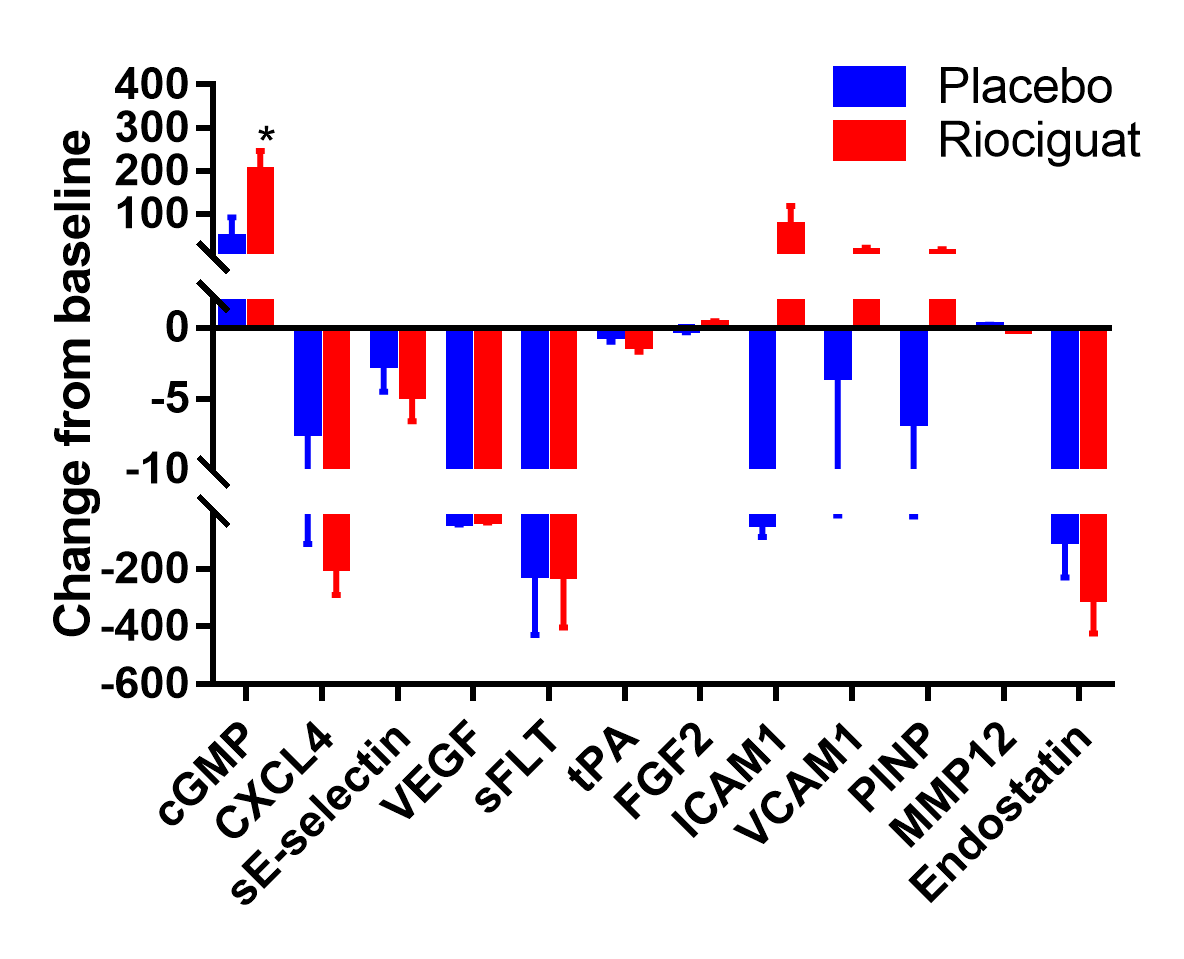
Results: We randomized 17 participants with SSc-DUs at 5 US centers between January 2017 and March 2018. Six participants in each group progressed transitioned to the open-label extension phase. Baseline characteristics were comparable between treatment groups, except participants randomized to PLA were older (mean 61 vs. 43 yrs) with longer disease duration (mean 17.5 vs. 7.1 yrs). At baseline, the mean (SD) NUB was 2.5 (2.0) in the PLA and 2.4 (1.4) in the RIO. No significant difference was observed between RIO and PLA in change from baseline to 16 weeks in NUB [treatment difference -0.24 favoring RIO, p=0.70; Figure 1]. In the open-label extension, participants in the RIO-RIO arm had complete healing of their DUs.There were no statistically significant treatment differences in secondary efficacy endpoints, except for the eating component of HAQ-DI (Table 1). All 17 participants reported ≥1 adverse event, with the vast majority being mild or moderate. Four participants experienced 5 serious AE (4 in RIO and 1 in PLA); none was considered related to study medication. Statistically significant elevations of cGMP were observed at 16 weeks [treatment difference 157.3 nM, p=0.05, Figure 2]; no other biomarkers showed statistically significant changes.
Conclusion: In participants with SSc-DU, treatment with RIO did not reduce the number of DU net burden compared with PLA. Open-label extension suggests that a longer duration is needed to promote DU healing, which needs to be confirmed within a new trial.
The negative results may reflect a small number of patients, low number of NUB at baseline, moderate-to-severe vasculopathy with long term disease, and the difficulty to recruit patients in the era of widespread use of PDE5 inhibitors.
To cite this abstract in AMA style:
Nagaraja V, Spino C, Tsou P, Domsic R, Lafyatis R, Frech T, Gordon J, Steen V, Khanna D. Multicenter Double-Blind, Proof-of-Concept, Randomized Placebo-Controlled Trial of Riociguat in Systemic Sclerosis-associated Digital Ulcers [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/multicenter-double-blind-proof-of-concept-randomized-placebo-controlled-trial-of-riociguat-in-systemic-sclerosis-associated-digital-ulcers/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multicenter-double-blind-proof-of-concept-randomized-placebo-controlled-trial-of-riociguat-in-systemic-sclerosis-associated-digital-ulcers/