Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE or lupus) is a clinically heterogeneous autoimmune disease, in which emerging evidence implicates the innate immune system, particularly monocytes and macrophages, in its pathogenesis.
Methods: Single cell RNA sequencing (scRNA-seq) analysis and cytokine multiplex with ELISA were performed on monocytes from
healthy individuals stimulated with lupus small nuclear ribonucleoprotein (snRNP) immune complex. DNA microarray studies were performed on monocytes stimulated with double-stranded DNA (dsDNA) and Ro immune complexes. Monocyte and macrophage populations in scRNA-seq data from lupus nephritis, acute cutaneous lupus and peripheral blood from lupus patients were interrogated. Imaging mass cytometry (IMC) was performed on kidney biopsies from patients with lupus nephritis.
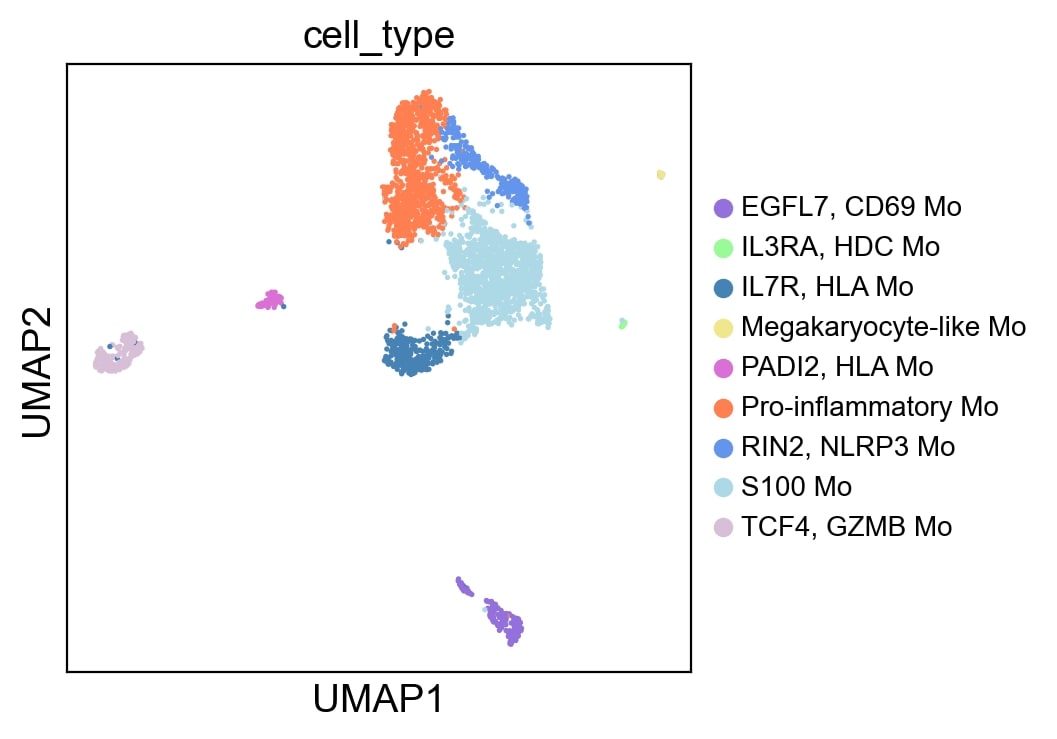
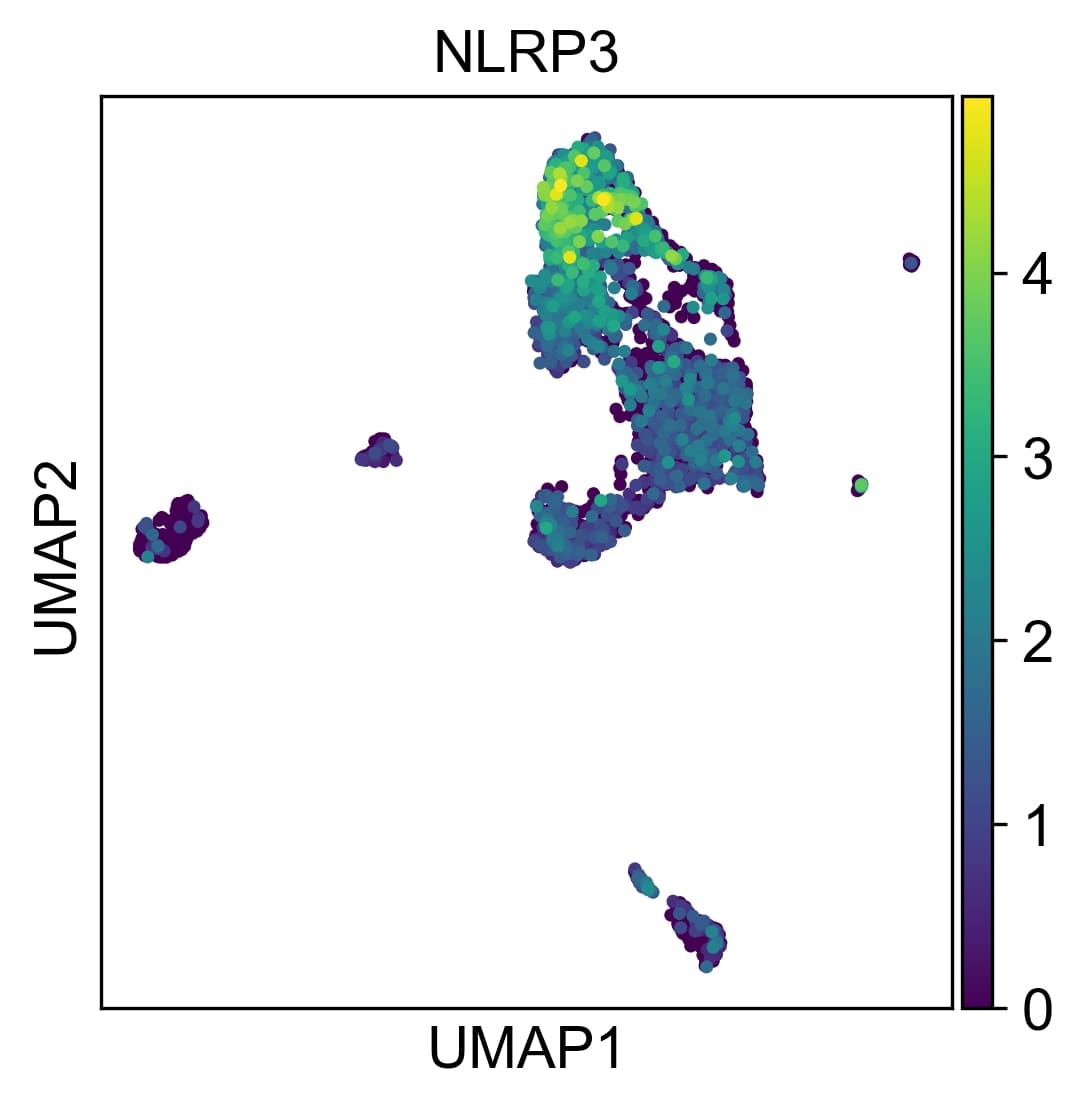
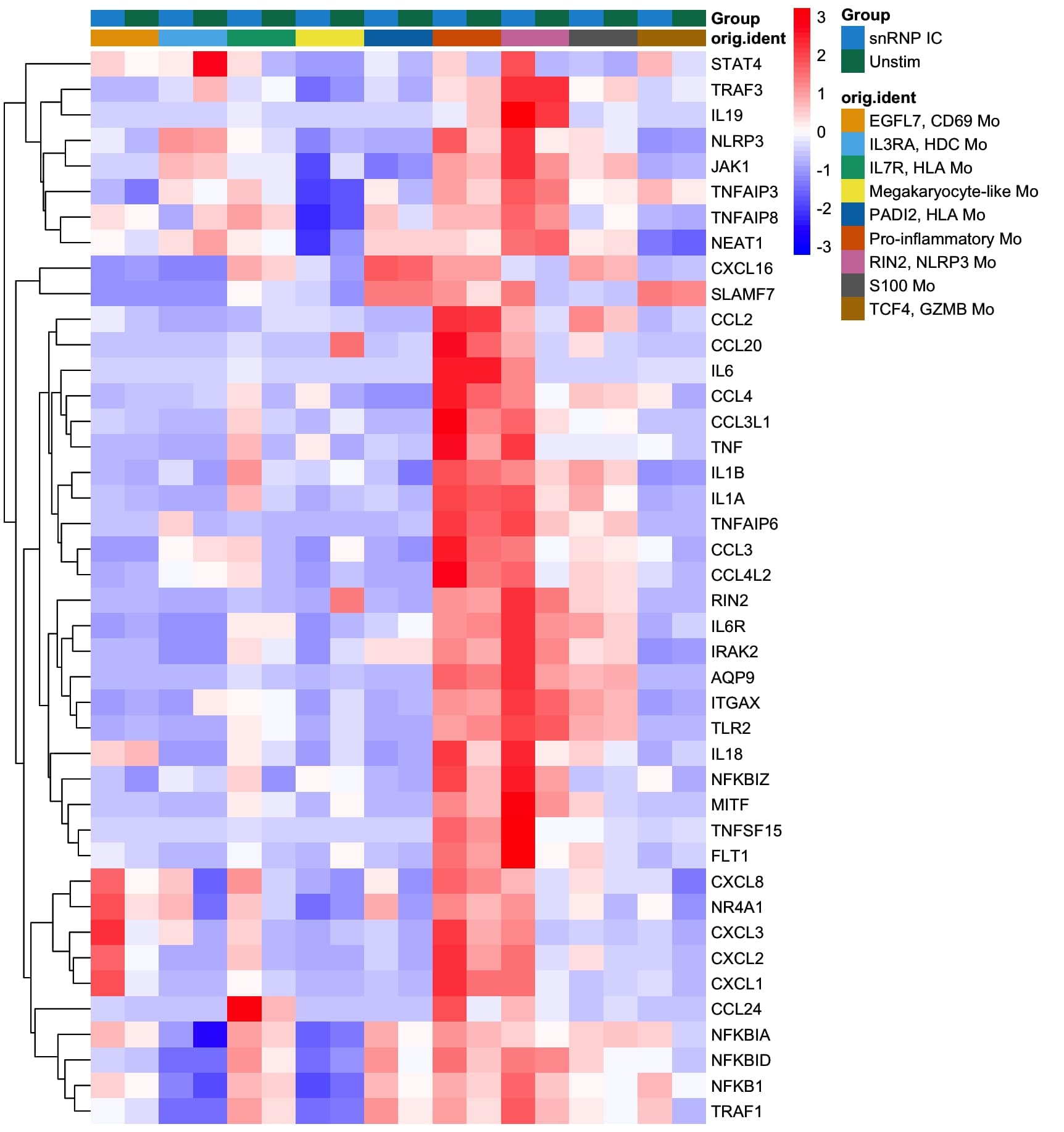
Results: Through scRNA-seq analysis of monocytes from healthy individuals stimulated with snRNP immune complex, we identified an expanded pro-inflammatory monocyte population characterized by upregulated expression of genes encoding pro-inflammatory cytokines (TNF, IL6, IL1A, IL1B, IL18), chemokines (CCL2, CCL3, CXCL1, CXCL2, CXCL3) and other molecules such as NLRP3, a main component of the NLRP3 inflammasome (Figure 1). Similar pro-inflammatory gene signatures from microarray analysis were observed in monocytes stimulated with dsDNA and Ro immune complexes. These pro-inflammatory transcriptomic changes were validated at the protein level by cytokine multiplex and ELISA. Interrogation of scRNA-seq data from lupus patients’ skin, kidney and peripheral blood revealed analogous expansion and activation of pro-inflammatory populations of mononuclear phagocytic cell populations, including monocytes and macrophages. Single cell communication studies demonstrated complex pro-inflammatory signaling by mononuclear phagocytic cells, targeting both adaptive and innate immune cells in acute cutaneous lupus and lupus nephritis. Imaging mass cytometry (IMC) of lupus nephritis biopsies demonstrated increased myeloid cell infiltration and NLPR3 expression in subjects who progressed to renal failure despite treatment. IMC spatial analysis identified interactions between NLRP3-expressing macrophages and B and T cells. Additionally, we found multiple lines of evidence implicating the NF-kB and JAK-STAT pathways as mediators of the pro-inflammatory transcriptomic and proteomic changes observed in monocytes stimulated with lupus immune complex and in the pro-inflammatory mononuclear phagocytic cell populations in lupus nephritis and acute cutaneous lupus.
Conclusion: Collectively, our findings support the critical pathogenic role of pro-inflammatory monocyte and macrophage populations, which develop in response to lupus immune complex stimulation, in the pathogenesis of SLE. Further, our multi-omic approach has also identified potential therapeutic targets.
To cite this abstract in AMA style:
Osmani L, Shin M, Lee S, Cai H, Seong W, Kim H, Yoo J, Mirabella A, Bracamonte W, Felix M, Ahn J, Park H, Young J, Shin J, Unlu S, Yoo N, Doherty E, Chen J, Li C, Sanchez-Zuno G, Valdez C, Tran T, Dong M, Kim S, Ko C, You S, Gomez J, Bucala R, Kang I. Multi-omic Profiling Identifies Pathogenic Pro-inflammatory Human Monocytes/Macrophages in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/multi-omic-profiling-identifies-pathogenic-pro-inflammatory-human-monocytes-macrophages-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multi-omic-profiling-identifies-pathogenic-pro-inflammatory-human-monocytes-macrophages-in-systemic-lupus-erythematosus/