Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Although mouse models of systemic lupus erythematosus (SLE) are useful proxies for human illness, they are heterogeneous, and publications about mouse SLE may not sufficiently emphasize differences between animals and humans with SLE. We examined recent literature to compare the criteria used to label mice as having SLE to phenotypes of human SLE.
Methods: Using “lupus AND animal NOT (review OR systematic review OR meta-analysis)” as the search terms, and filtering for full-text availability and English, we systematically reviewed studies published between July 3, 2013 and July 3, 2018, excluding review articles, papers from journals with impact factor (IF) < 2, and non-mouse animal studies. We recorded main mouse model groups (spontaneous, induced, transgenic/knockout, or combinations), sub-groups (MRL/lpr, NZB/W F1, pristane), and gender for each study; how lupus was defined in the model (clinical manifestations, immunohistochemistry, and serology); and how and when authors defined clinical and serological disease onset (< 8 weeks, 8-16weeks, >16weeks). Additionally, asked whether the title of the article easily informed the reader whether the study was of animals or humans.
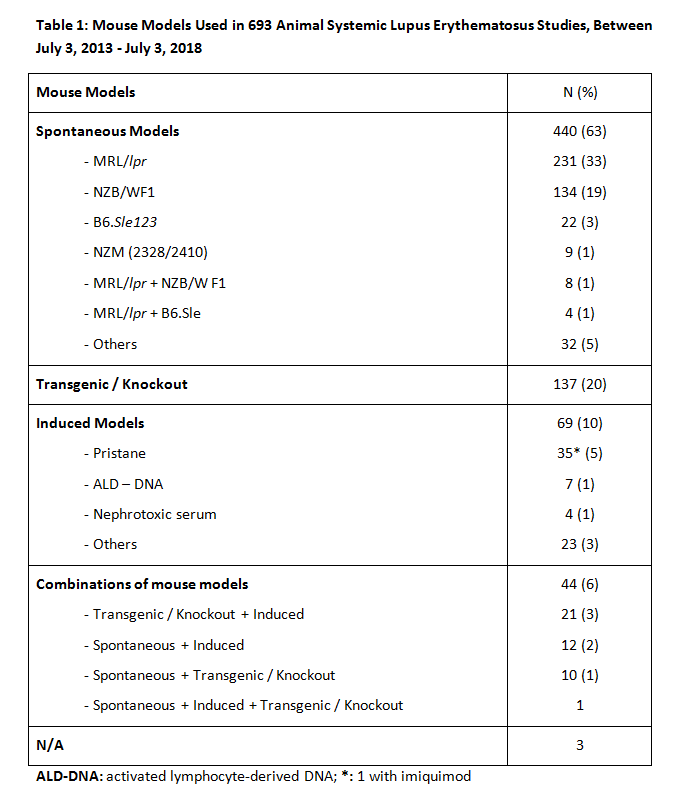
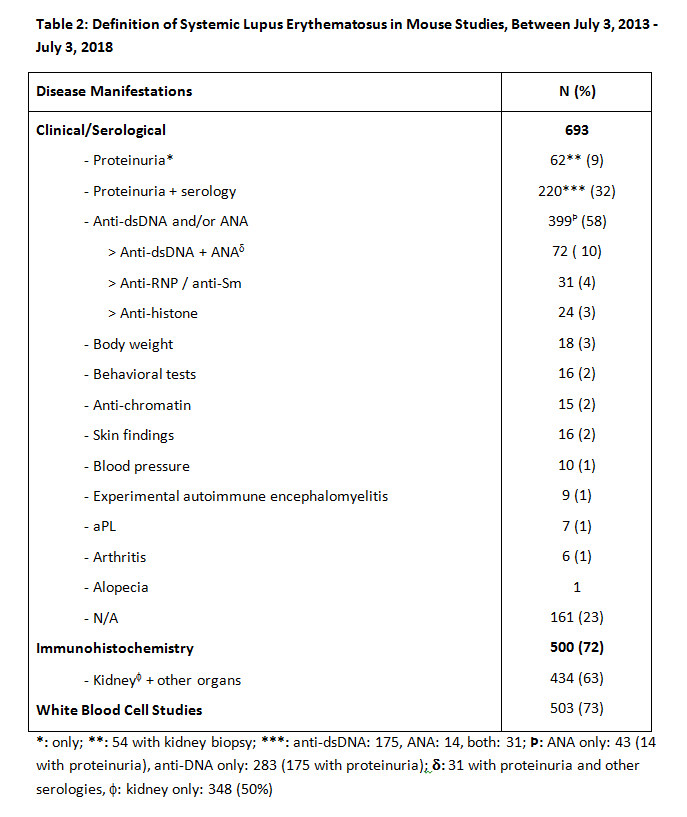
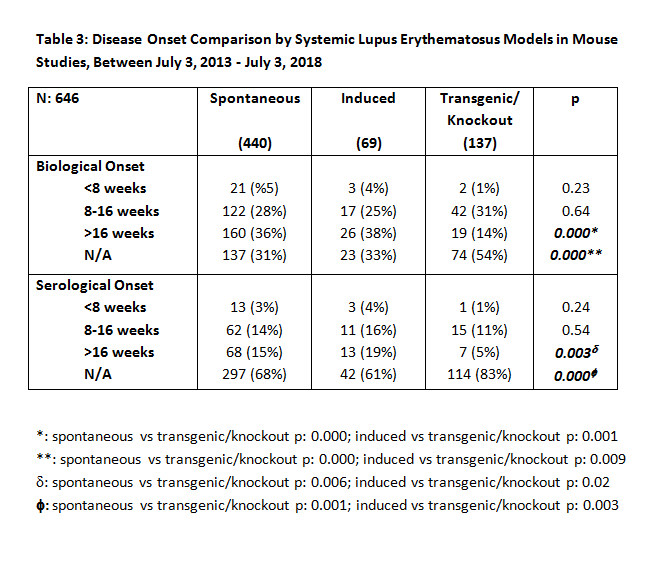
Results: Of 1572 articles identified, 693 were suitable for analysis. Table 1 presents mouse models used in 693 animal SLE studies. Four hundred forty (63%) used models of spontaneous heritable SLE (243 [35%] MRL/lpr and 147 [21%] NZB/W F1); 69 (10%) used induced models, of which 35 (5%) were pristane-induced; 138 (20%) used transgenic/knockout models; and 44 (6%) used combinations of models. Determinants of diagnosis, as shown in Table 2, were anti-DNA antibody and/or ANA autoantibody in 58% of studies, proteinuria in 41%, both serology and proteinuria in 32%, and immunohistochemistry in 72%. Of the latter, 500 (63%) included renal pathology. Other criteria included behavioral tests in 2%, skin manifestations in 2%, and arthritis in 1%. Only 64% of papers defined time of clinical and/or biological onset of illness (< 8 weeks, 4%; 8-16 weeks, 28%; >16 weeks 32%; not indicated, 36%), and only 30% defined time of serological onset (< 8 weeks, 3%; 8-16 weeks, 14%; >16 weeks, 14%; not indicated, 70%). Table 3 compares disease onset, clinical, biological, and serological definitions of the mouse model groups, excluding studies that used combinations of these models. Seventy-one percent of papers’ titles mentioned SLE; but in only 38% of those, and 44% of all papers, did the titles indicate that the study concerned animal rather than human SLE.
Conclusion: Publications about mouse SLE use the term SLE in ways that do not easily translate to human SLE. SLE is defined by histopathology in most mouse studies and by autoantibodies in slightly more than half. Many studies do not define sex, age of onset, or disease activity of diagnosed animals. While mouse models play an important role in understanding human lupus, the different criteria used to define mouse and human lupus may mislead readers seeking information about SLE who are unfamiliar with details of mouse models.
To cite this abstract in AMA style:
Sevim E, Jia L, Fernandez D, Lockshin M. Mouse SLE Studies Do Not Describe Human SLE [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/mouse-sle-studies-do-not-describe-human-sle/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mouse-sle-studies-do-not-describe-human-sle/