Session Information
Date: Monday, November 18, 2024
Title: Plenary III
Session Type: Plenary Session
Session Time: 9:00AM-10:30AM
Background/Purpose: There is growing awareness that immunotherapy-related adverse events (iRAE) from immune checkpoint inhibitor (ICI) therapy are being correlated with treatment outcomes of various cancers. Patients with pre-existing autoimmune disease (AID) continue to be excluded from ICI clinical trials. It is important to understand whether patients with pre-existing AID respond differently to ICI therapy and thereby have a different mortality risk or increased risk of iRAE compared to patients without AID. Several recent studies suggest equivalent survival rates in patients with AID [1,2,3], though the largest study by Tang et al. was limited by short follow-up duration. The objective of this study was to assess mortality risk in a national cohort of patients with pre-existing AID being treated with ICI therapy.
Methods: We conducted a cohort study using the TriNetX Diamond network, a large, multi-center network of U.S. electronic health records. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify patients with pre-existing AID on anti-programmed death-1/programmed death ligand-1 (PD-1/PD-L1) immunotherapy for the most common malignancies treated with ICI therapy (C34: bronchus and lung, C15-26: digestive organs, C43: melanoma, and C64-68: urinary tract). We analyzed rates of mortality following ICI initiation. Propensity score matching included factors to adjust for demographics and co-morbidities. Kaplan-Meier analysis and Cox proportional hazards models were used to estimate the probability of the outcome of interest.
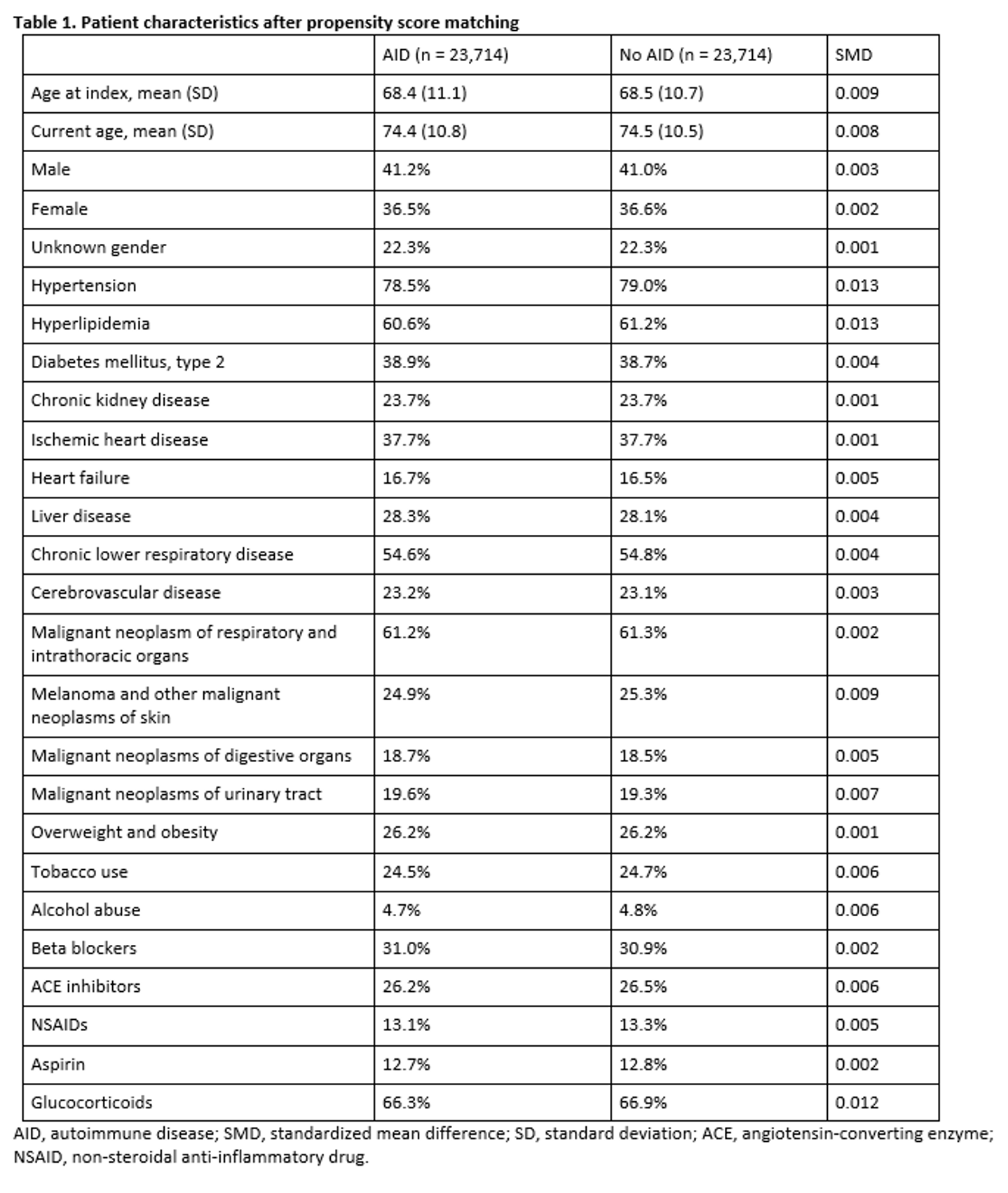
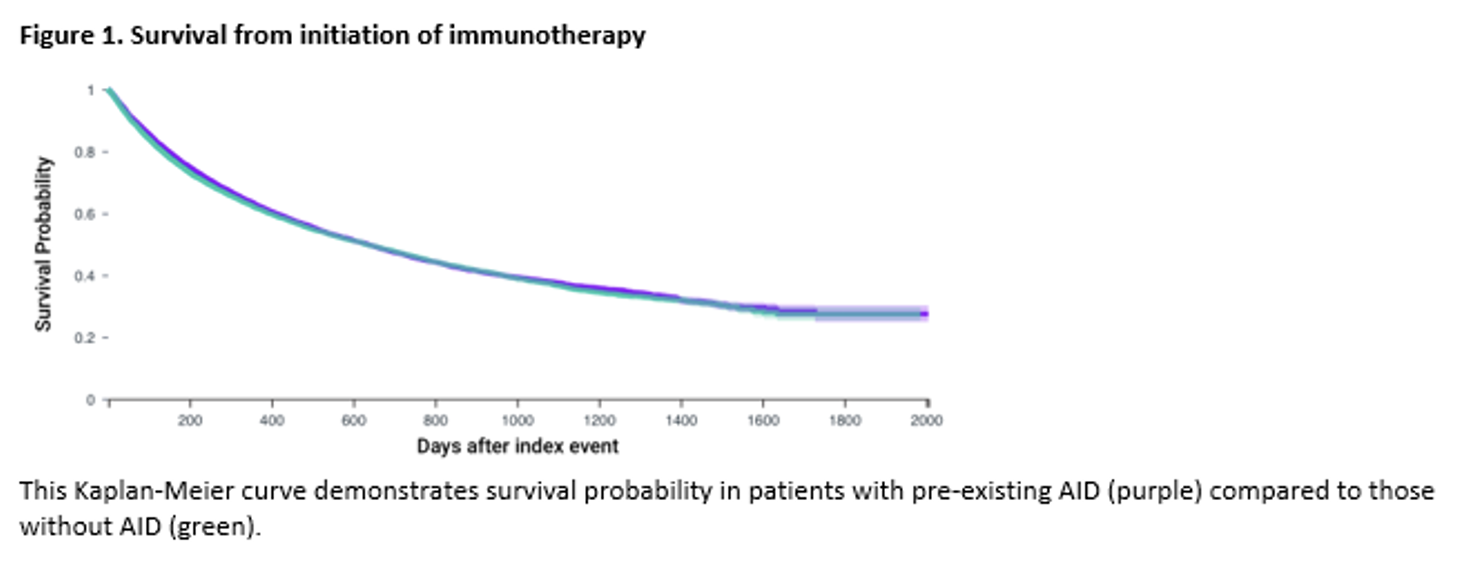
Results: A total of 25,153 patients on ICIs with known AID and 78,547 patients without known AID were included. Prior to propensity score matching, the rate of mortality at last follow-up was 40.0% in those with known AID and 38.1% in those without pre-existing AID with hazard ratio (HR) for mortality of 1.07 (95% CI 1.05-1.10). The AID patient population had notably higher rates of cardiovascular comorbidities when compared to those without known AID including higher rates of type 2 diabetes mellitus (42.0% vs 24.8%, respectively), chronic kidney disease (25.6% vs 15.5%, respectively), and ischemic heart disease (39.2% vs 28.4%). After 1:1 propensity score matching, there were two cohorts of 23,714 patients (Table 1). No significant mortality difference was observed between these groups with rates of mortality at last follow-up of 39.8% in the AID population compared to 40.2% in the population without AID with observed HR for mortality of 0.97 (95% CI 0.94-1.00) with Kaplan-Meier survival curve depicted in Figure 1.
Conclusion: This study of a large population of patients receiving ICI therapy provides further evidence that there is no significant difference in mortality risk in patients with pre-existing AID compared to those without.
References
1. PMID: 35797031
2. PMID: 35188215
3. PMID: 37841635
4. PMID: 36636443
To cite this abstract in AMA style:
Challener G, sheng-kai ma k, Kohler M, Yokose C, Choi H. Mortality in Patients with Pre-existing Autoimmune Disease on Immune Checkpoint Inhibitor Therapy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/mortality-in-patients-with-pre-existing-autoimmune-disease-on-immune-checkpoint-inhibitor-therapy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mortality-in-patients-with-pre-existing-autoimmune-disease-on-immune-checkpoint-inhibitor-therapy/