Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
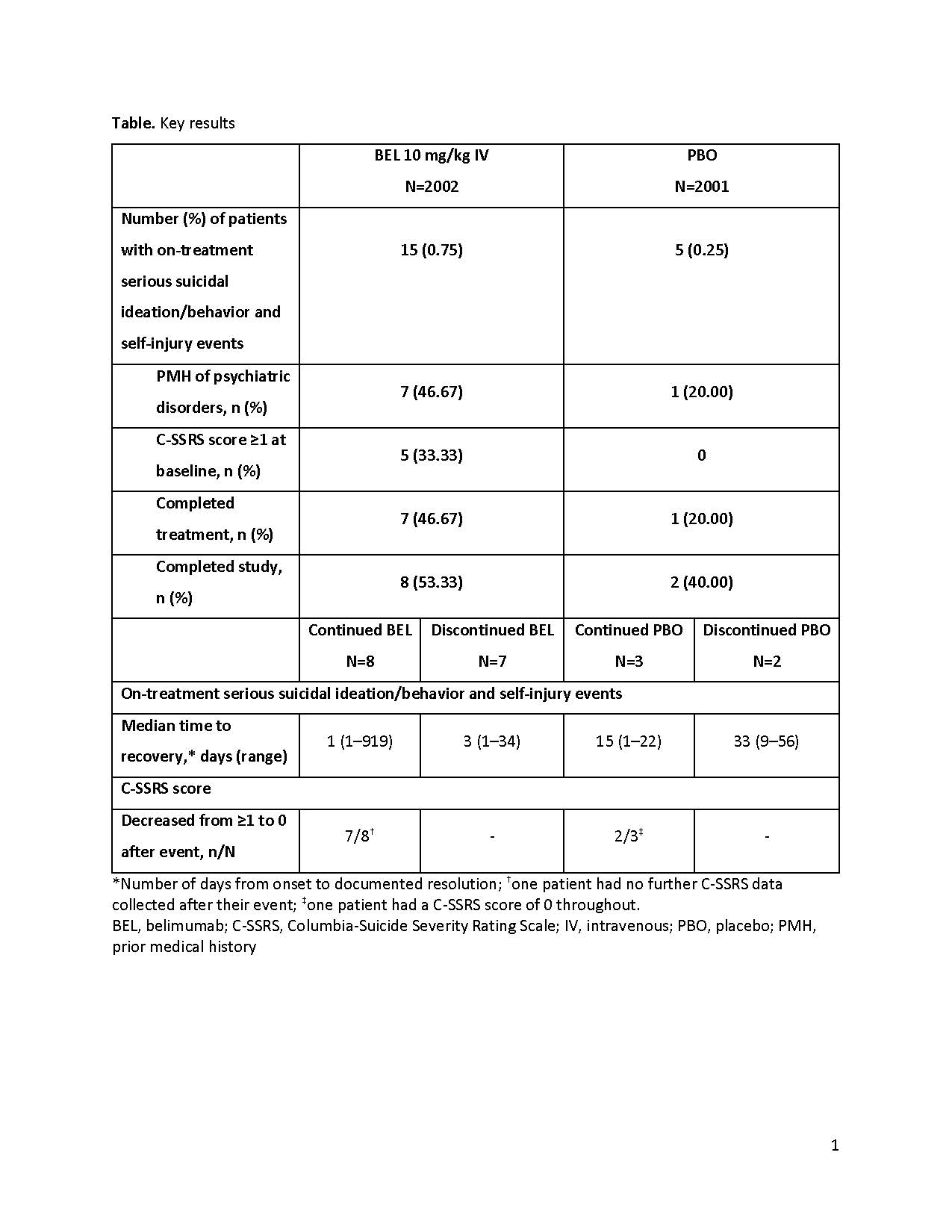
Background/Purpose: Intravenous (IV) belimumab (BEL) is approved in patients ≥5 years of age with active systemic lupus erythematosus (SLE). Results of the BASE study (the largest SLE clinical study to date: n=4003), demonstrated similar rates of on-treatment all-cause mortality, infection and malignancy adverse events of special interest (AESI) between BEL and placebo (PBO). However, infrequent but higher incidences of other AESI including serious psychiatric events, fatal infections and hypersensitivity reactions occurred with BEL than PBO.1 This analysis provides a post hoc descriptive summary of the disposition of patients who reported on-treatment serious suicide/self-injury events.
Methods: BASE (GSK Study BEL115467; NCT01705977) randomized adults with SLE (1:1) to receive BEL 10 mg/kg IV or PBO, plus standard SLE therapy, administered on Weeks 0, 2, 4, then monthly until Week 48. Rates of mortality and other pre-specified AESI, including serious psychiatric events and suicidality by Columbia-Suicide Severity Rating Scale (C-SSRS), were assessed on-treatment. The following were summarized post hoc for on-treatment serious suicide/self-injury events: time to onset, prior medical history (PMH), treatment completion and study completion, whether investigational product (IP) was taken after these events occurred, time to recovery, the C-SSRS score profile and outcome.
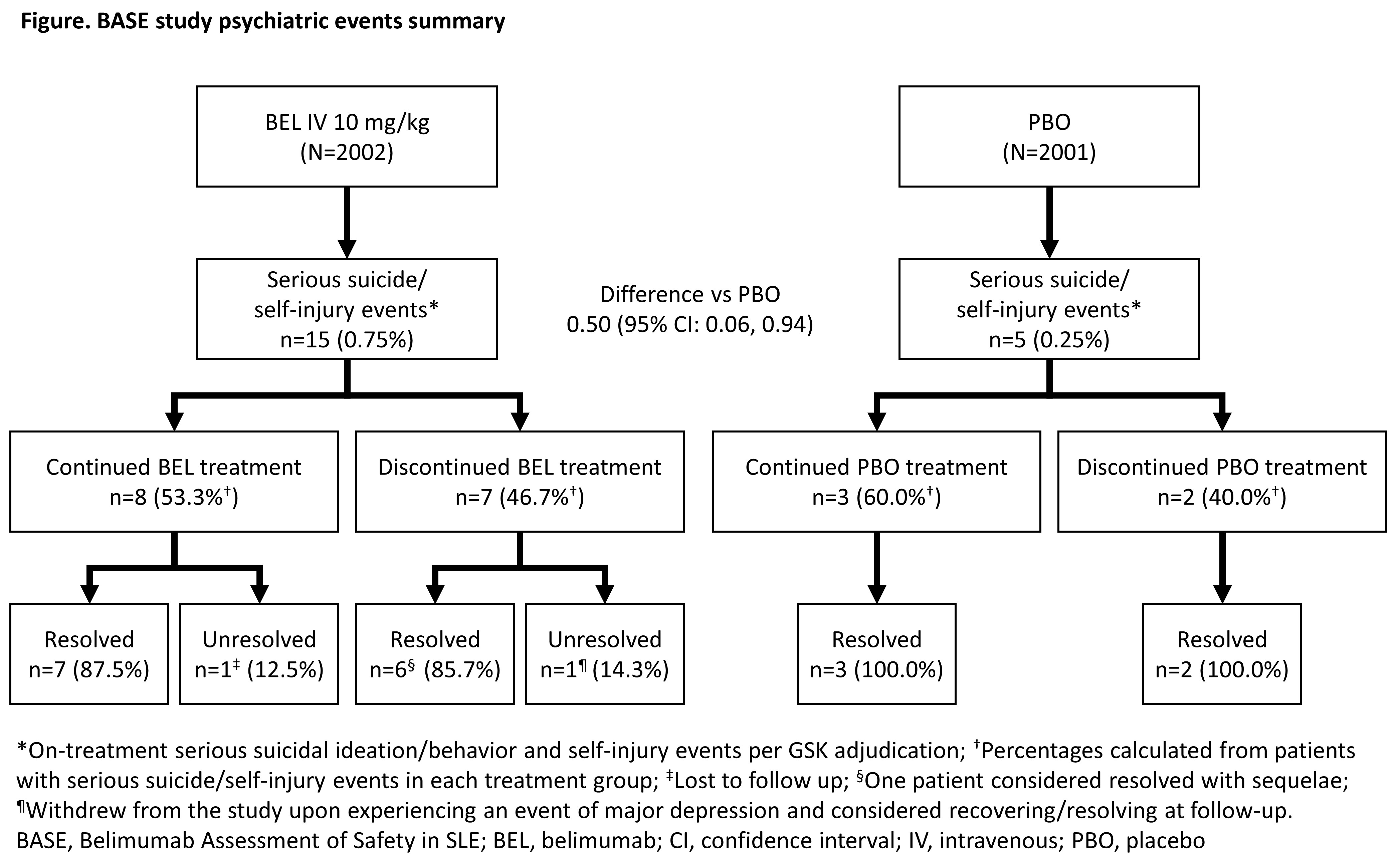
Results: There were 20 on-treatment serious suicidal/self-injury events (per sponsor adjudication), reported in 20 patients: 15 (0.75%) BEL and 5 (0.25%) PBO (post hoc difference vs PBO [95% confidence interval] 0.50 [0.06, 0.94]: Figure). All patients who experienced an event were female (aged 18–66 years, weight 40.5–99.3 kg, and mainly white [n=9] or black/African American [n=6]); 8/20 patients had a PMH of psychiatric disorder (Table). The median time to onset of the event was 205 days for BEL and 204 days for PBO. Approximately half (8/15) of the BEL patients and 3/5 PBO patients continued IP after these events occurred. The median time to recovery (number of days from onset to documented resolution) was shorter in those who did versus did not receive further IP, respectively, and lower in the BEL group vs the PBO group overall (Table). For the BEL and PBO groups who received further IP after the event, the C-SSRS score generally fell to 0 after the event (Table) and did not rebound, indicating resolution without further suicidal ideation and/or behavior during the study. Overall, 18/20 events were considered resolved and 0 patients experienced a recurrent event (Figure). No suicide-related deaths were reported.
Conclusion: An imbalance was observed with higher on-treatment serious suicidal ideation/behavior and self-injury events with BEL than PBO. These results are consistent with previously published data. The median time to onset of these events was similar between BEL and PBO, with the majority resolving with no sequelae, regardless of whether IP was discontinued or not. Overall the benefit:risk profile of belimumab remains positive.
1Sheikh S, et al. Arthritis Rheumatol 2019;71(S10):Abstract 0858
To cite this abstract in AMA style:
Sheikh S, Acayaba de Toledo R, Geraldino-Pardilla L, Harris J, Kurrasch R, Liu A, Maksimowicz-McKinnon K, Quasny H, Roth D, Soto L, Punwaney R. Mortality and Adverse Events of Special Interest in Adult Patients with Systemic Lupus Erythematosus Receiving Intravenous Belimumab: A Post Hoc Descriptive Summary of Serious Psychiatric Events [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/mortality-and-adverse-events-of-special-interest-in-adult-patients-with-systemic-lupus-erythematosus-receiving-intravenous-belimumab-a-post-hoc-descriptive-summary-of-serious-psychiatric-events/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mortality-and-adverse-events-of-special-interest-in-adult-patients-with-systemic-lupus-erythematosus-receiving-intravenous-belimumab-a-post-hoc-descriptive-summary-of-serious-psychiatric-events/