Session Information
Date: Monday, November 14, 2022
Title: RA – Treatment Poster IV
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: A Treat-to-Target approach (T2T) is broadly considered to lead to better clinical outcomes and recommended in patients with RA. However, very few studies have analyzed the effect of T2T on radiographic progression, and any such studies have provided inconsistent results. Our aim was to investigate whether meticulously following a treat-to-target (T2T)-strategy in daily clinical practice leads to lower radiographic progression in RA.
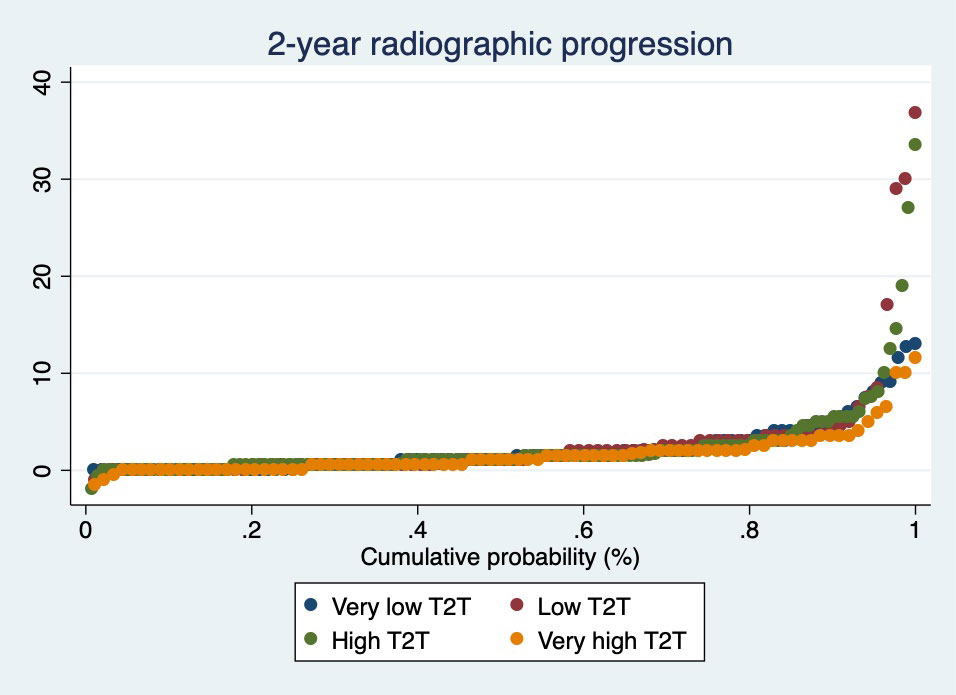
Methods: Patients from the multicenter RA-BIODAM cohort with ≥2 consecutive visits with radiographs available were included. In RA-BIODAM patients were enrolled as they were initiating a new csDMARD/bDMARD treatment were followed-up with the intention to benchmark and intensify treatment. The primary outcome of this analysis was the change in Sharp-van der Heijde score (SvdH, 0-448), assessed every 6 months, using average scores from 2 readers (scores with known chronological order). Following a DAS44-T2T remission strategy, which was defined at each 3-month visit, was the main variable of interest. Patients were categorized based on the proportion of visits in which T2T was followed according to our definition: very low (≤40% of the visits, low ( >40%, < 62.5%), high (≥62.5%, ≤75%) and very high ( >75%). Radiographic progression at 2 years was visualized across groups by cumulative probability plots. Per 3-month interval T2T could be followed zero, one or two times (in a total of 2 visits). Associations between the number of visits with T2T in an interval and radiographic progression, both in the same and in the subsequent 6-month interval, were analysed by generalised estimating equations, adjusted for age, gender, disease duration and country.
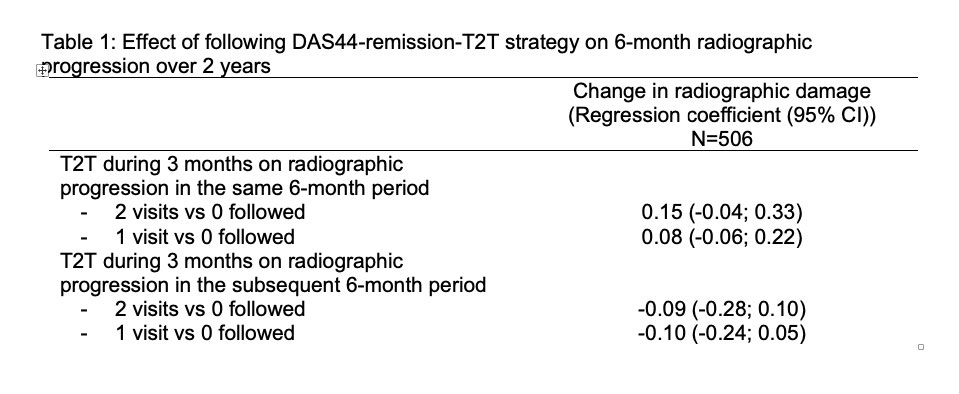
Results: In total, 511 patients were included (mean (SD) age: 56 (13) years; 76% female). After 2 years, patients showed on average 2.2 (4.1) units progression (median:1 unit). Mean (SD) 2-year progression was not significantly different across categories of T2T: very low: 2.1 (2.7)-units; low: 2.8 (6.0); high: 2.4 (4.5), very high: 1.6 (2.2) (Figure). Meticulously following-up T2T in a 3-month interval neither reduced progression in the same 6-month interval (parameter estimates (for yes vs no): +0.15 units (95%CI: -0.04 to 0.33) for 2 vs 0 visits; and +0.08 units (-0.06;0.22) for 1 vs 0 visits) nor did it reduce progression in the subsequent 6-month interval (Table).
Conclusion: In this daily practice cohort, more meticulously following T2T principles did not result in more reduction of radiographic progression than a somewhat more liberal attitude toward T2T. One possible interpretation of these results is that the intention to apply T2T already suffices and that a more stringent approach does not further improve outcome.
To cite this abstract in AMA style:
Ramiro S, Landewé R, van der Heijde D, Sepriano A, FitzGerald O, Østergaard M, Homik J, Elkayam O, Thorne C, Larche M, Ferraccioli G, Backhaus M, Boire G, Combe B, Schaeverbeke T, Saraux A, Dougados M, Rossini M, Govoni M, Sinigaglia L, Cantagrel A, Allaart C, Barnabe C, Bingham III C, van Schaardenburg D, Hammer H, Dadashova R, Hutchings E, Paschke J, Maksymowych W. More Meticulously Following Treat-to-target in RA Does Not Lead to Less Radiographic Progression: A Longitudinal Analysis in BIODAM [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/more-meticulously-following-treat-to-target-in-ra-does-not-lead-to-less-radiographic-progression-a-longitudinal-analysis-in-biodam/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/more-meticulously-following-treat-to-target-in-ra-does-not-lead-to-less-radiographic-progression-a-longitudinal-analysis-in-biodam/