Session Information
Date: Monday, November 11, 2019
Title: Metabolic & Crystal Arthropathies Poster II: Clinical Trials & Basic Science
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Long term gout treatment focuses on reducing sUA levels, thus allowing MSU crystals to dissolve. Rapid dissolution of MSU crystals during initial phase of urate lowering therapy (ULT) is associated with an increased incidence (75%) and frequency (2.1 flares/patient/month)* of acute gout flares contributing to poor treatment compliance. During ULT initiation, colchicine, NSAIDs or corticosteroids are used for gout flare prophylaxis. Pegylated uricases are therapies for the treatment of chronic gout. However, their efficacy and safety are limited by induction of anti-drug antibodies (ADA). SEL-212 is a novel combination product consisting of pegadricase co-administered with proprietary ImmTOR tolerogenic nanoparticles. We report here data on gout flares from a Phase 2 study in symptomatic gout patients.
Methods: Patients with symptomatic gout (≥1 tophus, gout flare within 6 months or gouty arthropathy) and elevated serum uric acid (sUA) ≥6 mg/dL were randomized to receive doses of pegadricase (0.2 mg/kg or 0.4 mg/kg) alone or in combination with ImmTOR (0.05 to 0.15 mg/kg). Treatments were administered intravenously monthly for 3 cycles of SEL-212, followed by 2 additional cycles with pegadricase alone or SEL-212. Safety, tolerability, sUA, and ADAs were monitored. Here we report on the patients that received 5 combination doses of ImmTOR and pegadricase.
94% (49/52) of all randomized patients received premedication for gout flare prevention as per standard of care in the form of colchicine (1.2 mg as loading dose, 0.6 mg QD for the remainder of their participation), ibuprofen, or equivalent dose of NSAID.
Results: Demographics of 46 patients treated with 28-day cycles x5 combination doses of ImmTOR and pegadricase as compared to 6 patients treated with pegadricase alone were 23-70 years old vs. 41-64 years old (mean 53.6 vs. 51.8 years), male 97.8% vs. 100%, and white 73.9%. vs. 33.3%. The mean BMI at baseline was 34.5 vs. 38.9 kg/m2. 71.7% vs. 100% of patients were obese with mean duration of established or symptomatic gout was 12.5 vs. 12.8 years. 43 patients were evaluable for gout flare analysis.
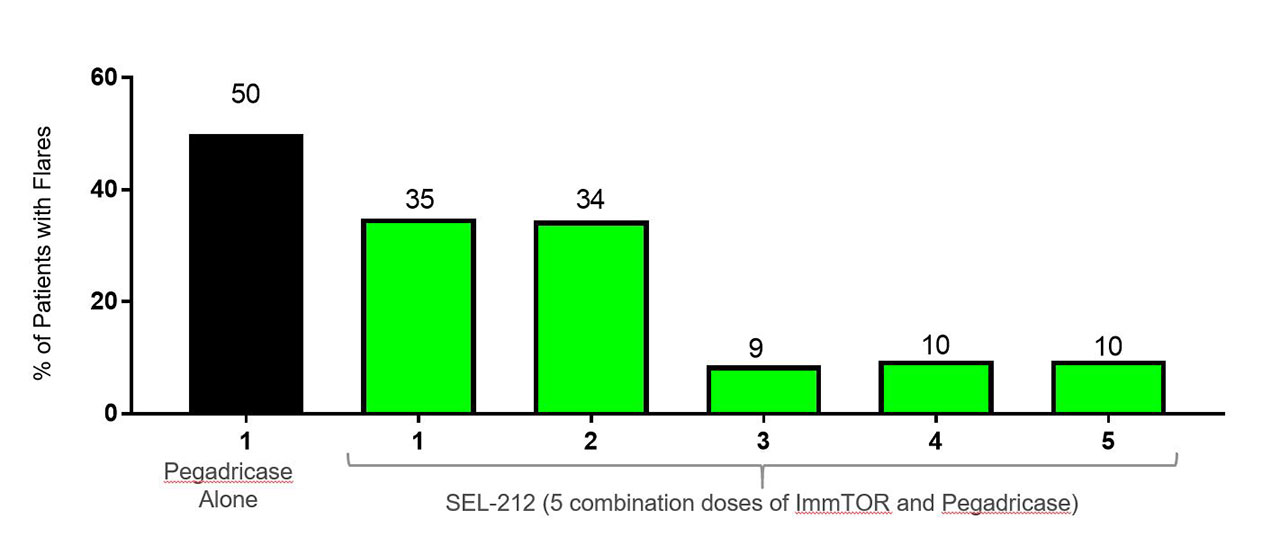
In the first month 50% of patients who received pegadricase alone had flares compared to 35% receiving SEL-212. There were no patients with initial flares after second month of treatment.
Data analyzed as combined months in patients receiving SEL-212, showed flare incidence of 37.0% (months 1-3) and 13.6% (months 4-5), flare frequency was 0.83 flares/patient (months 1-3) and 0.27 flares/patient (months 4-5). Mean duration of the gout flares was 6.1 days, with majority of the gout flares (97.7%) being categorized as mild/moderate, with 2.3% (n=1) noted as severe in intensity. Adjustments to gout flare prevention medication were not required for 69.6% of the patients. No gout flares resulted in patient discontinuation or were reported as a study drug related serious adverse event.
Conclusion: Monthly dosing of ImmTOR combined with pegadricase has been well-tolerated and has a lower incidence of flares at the initiation of therapy relative to pegylated uricases alone and the reported ULT flare incidence, and the effect persists over the duration of therapy.
*JAMA. 2011;306(7):711-720
To cite this abstract in AMA style:
Azeem R, Park J, Plotkin H, Kivitz A, Johnston L, Kishimoto T, Smolinski S, DeHaan W. Monthly Dosing of ImmTOR Tolerogenic Nanoparticles Combined with Pegylated Uricase (Pegadricase) Enables Sustained Reduction of Acute Gout Flares in Symptomatic Gout Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/monthly-dosing-of-immtor-tolerogenic-nanoparticles-combined-with-pegylated-uricase-pegadricase-enables-sustained-reduction-of-acute-gout-flares-in-symptomatic-gout-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/monthly-dosing-of-immtor-tolerogenic-nanoparticles-combined-with-pegylated-uricase-pegadricase-enables-sustained-reduction-of-acute-gout-flares-in-symptomatic-gout-patients/