Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Baricitinib is an oral selective Janus kinase (JAK) 1 and JAK2 inhibitor. In the Phase II, 24-week, randomized, placebo-controlled, double-blind study JAHH (NCT02708095), once-daily baricitinib resulted in significant clinical improvements in patients with active SLE receiving standard background therapy, compared with placebo.1 We characterized the molecular and cellular immune pathways impacted by baricitinib in SLE.
Methods: A total of 314 patients were randomized 1:1:1 to receive once-daily placebo, baricitinib 2-mg, or baricitinib 4-mg for 24 weeks in study JAHH. Patients were 18 years of age or older, had a diagnosis of SLE, and had active disease involving skin or joints. Whole blood samples were obtained from patients in JAHH at baseline and weeks 2, 4, 12, and 24, and cellular and serologic immune biomarkers were measured using flow cytometry and immunochemistry assays. RNA was isolated from whole blood and analyzed using Affymetrix HTA2.0 array and quantitative PCR. Data were summarized to transcript level and analyzed using a mixed effects model on a log2 transformed response with multiplicity correction. Clinical and immunologic characteristics were compared between subgroups defined using molecular profiling.
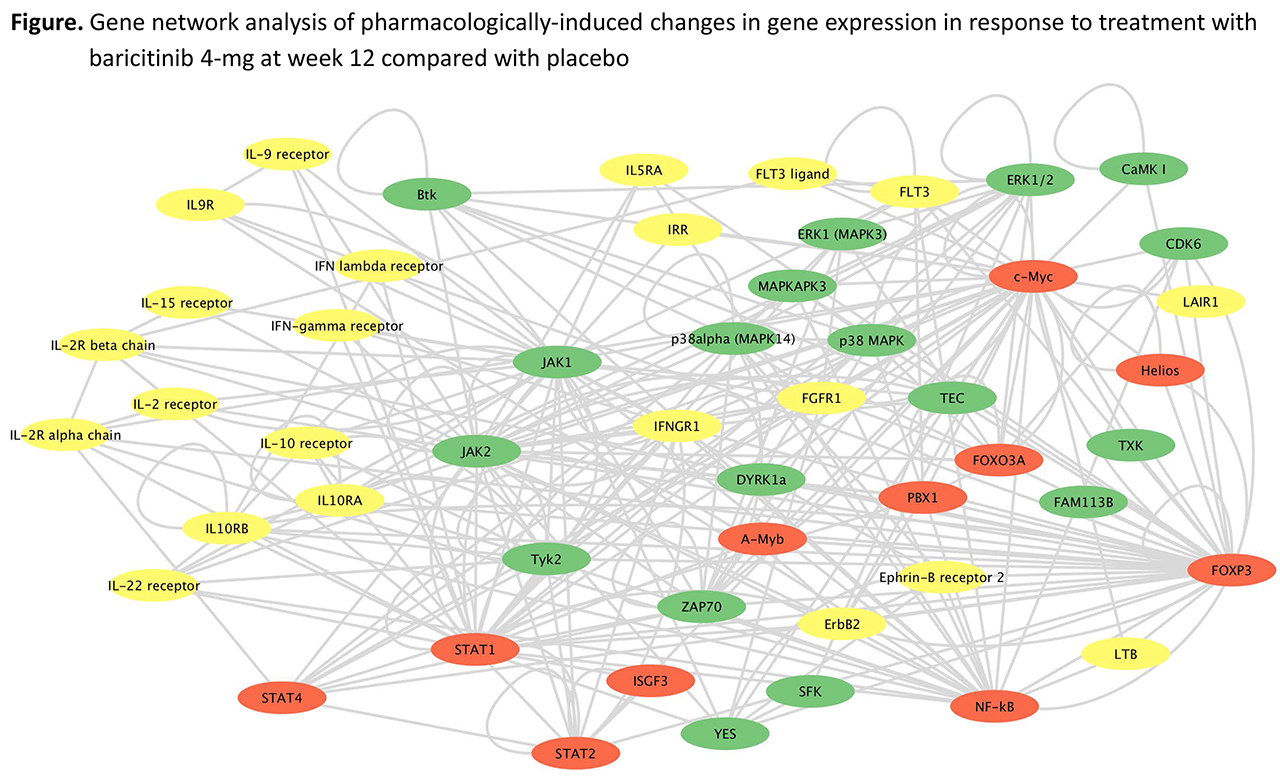
Results: At baseline, the IFN signature negatively correlated with complement (C) 3 and C4, and positively correlated with serum immunoglobulin (Ig) G, anti-dsDNA, anti-Sm, anti-RNP, anti-Ro 52/SSA, anti-Ro 60/SSA, and anti-La/SSB in patients with active SLE. These correlations constituted a distinctive subgroup, or endotype. Serum autoantibodies and complement were not altered by baricitinib treatment, suggesting that the mechanism of action of baricitinib in SLE may be primarily through an anti-inflammatory effect. Gene expression profiling demonstrated that there was an elevation of STAT1, STAT2, and multiple IFN responsive genes at baseline in patients with SLE. Statistical and gene network analysis demonstrated that baricitinib treatment reduced the expression of functionally interconnected genes involved in SLE including STAT1, STAT2, and STAT4, and multiple IFN responsive genes (Figure).
Conclusion: Baricitinib treatment reduced the expression of STAT1, STAT2, and IFN responsive genes in patients with SLE. Gene network analysis revealed that baricitinib treatment reduced the expression of a network of genes associated with JAK/STAT pathways, cytokine signaling, and SLE pathogenesis, suggesting that baricitinib effects may be mediated through pharmacologic impact on multiple immune pathways.
1Wallace DJ et al. Lancet. 2018;392:222-231.
To cite this abstract in AMA style:
Dörner T, Tanaka Y, Petri M, Smolen J, Wallace D, Dow E, Fantini D, Higgs R, Rocha G, Crowe B, Benschop R, Abel A, Byers N, Silk M, de Bono S, Hoffman R. Molecular Profiling Identifies Immunologic Subgroups and Informs Mechanism of Action of Baricitinib in SLE [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/molecular-profiling-identifies-immunologic-subgroups-and-informs-mechanism-of-action-of-baricitinib-in-sle/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/molecular-profiling-identifies-immunologic-subgroups-and-informs-mechanism-of-action-of-baricitinib-in-sle/