Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Reliable and effective treatments for refractory cutaneous lupus erythematosus (CLE) remained elusive until the arrival of anifrolumab, a monoclonal antibody targeting the type I interferon receptor subunit 1. In this study, we conducted high-resolution spatial and single-cell analysis from paired lesional and non-lesional skin samples of CLE patients pre- and post-anifrolumab treatment to gain insights into the immunopathogenesis of CLE and anifrolumab-induced changes in the cellular milieu. Our initial investigation highlighted significant changes in the spatial organization of immune cells, particularly in regions with increased expression of genes activated by type I interferon.
Methods: Punch biopsies of lesional and non-lesional skin from patients with refractory CLE were obtained prior to and three months after treatment with anifrolumab. We analyzed these formalin-fixed, paraffin-embedded (FFPE) skin biopsies using a single-cell gene expression method (Chromium Single Cell Gene Expression Flex) and a high-resolution spatial transcriptomics technique (Xenium In Situ), sequencing 27 and 17 skin biopsies respectively. This comprehensive approach facilitated the identification and spatial characterization of 53 different cell types.
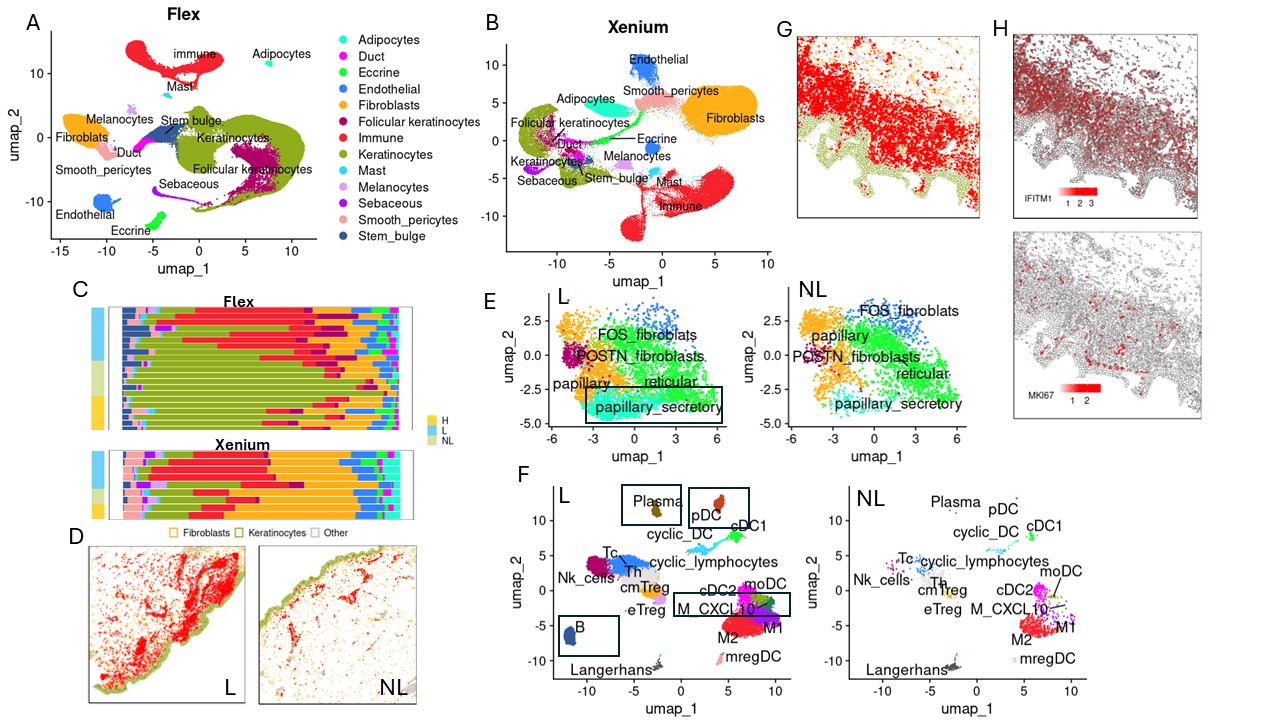
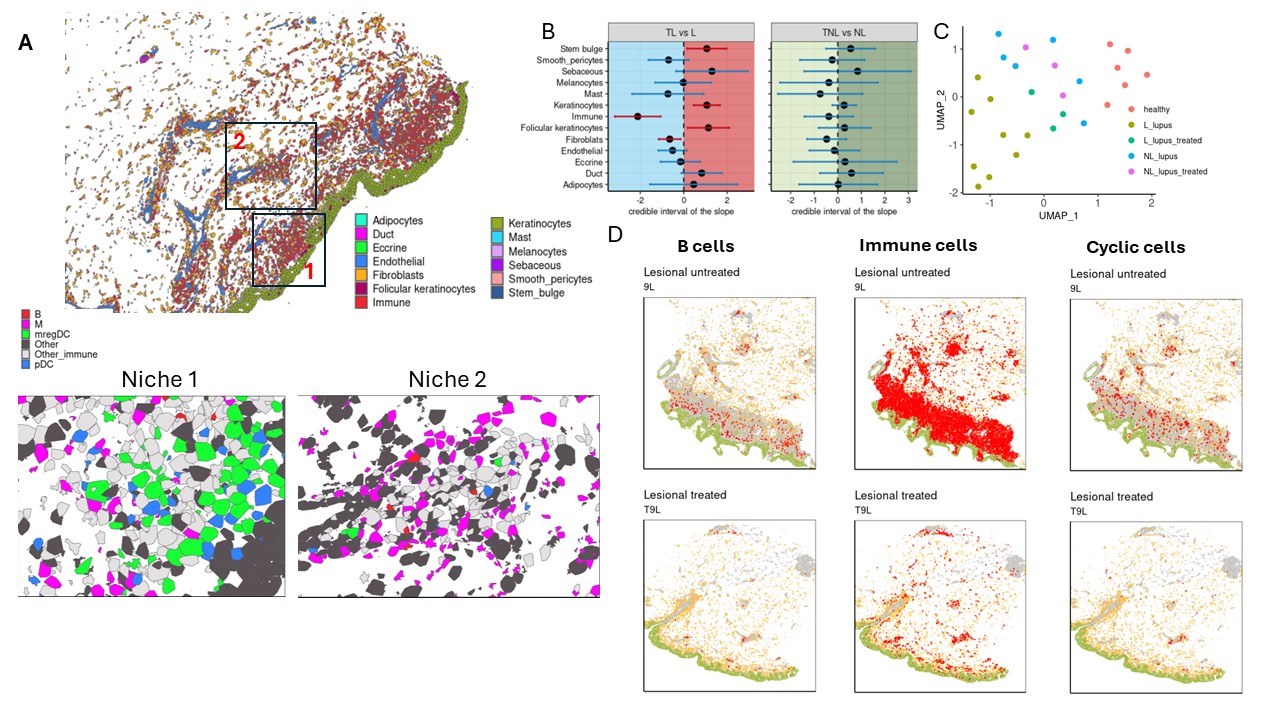
Results: Comparing single cell data from lesional and non-lesional skin biopsies revealed a significant increase in immune cell infiltration, which correlated with elevated levels of cells exhibiting a type I interferon response and proliferative phenotypes (Fig1, A-H). Additionally, pro-inflammatory clusters, including CXCL10-expressing macrophages and cytokine-enriched papillary fibroblasts, emerged in lesional skin (Fig1, E-F). Cell composition analysis demonstrated prevalence of plasmacytoid dendritic cells (pDCs), B cells, plasma cells, and CXCL10+ macrophages exclusively in lesional skin (Fig1, E-F). Spatial mapping across 17 skin biopsies unveiled two distinct clusters of immune aggregates in CLE lesional skin: one highly enriched mregDCs and pDCs occurring near basal keratinocytes, and another characterized by a predominance of macrophages located perivascularly (Fig2, A). Furthermore, analysis of spatial and single cell data from patients treated with anifrolumab demonstrated that reduced type I interferon signaling led to the disappearance of disease-associated clusters and depletion of spatially-organized immune aggregates (Fig2, B-D).
Conclusion: Inhibition of type I interferon signaling induces a shift of the lesional skin transcriptome towards the non-lesional phenotype, characterized by resolution of pro-inflammatory clusters and spatially-distinct immune aggregates. Notably, despite clinical improvement, treatment with anifrolumab did not result in the conversion of either the lesional or non-lesional skin transcriptome into the healthy phenotype. Further research is needed to elucidate the specific molecular response mechanisms underlying the superiority of anifrolumab over other therapies targeting dysregulated interferon signaling.
(B) A plot of estimates of differential composition analysis for major cell types between treated and untreated lesional and non-lesional skin samples. The error bars represent 95% confidence intervals. The error bar is blue if the composition of the target cell type is insignificant and red if significant. (C) UMAP of Multicellular Factor Analysis factor scores showing the variability of all cell types captured by single cell Flex sequencing. (D) Cell type map from Xenium data in lesional skin before and after treatment. Colors: green – keratinocytes, yellow – fibroblasts, red – B cells/immune cells/cells with a proliferative phenotype.
To cite this abstract in AMA style:
Anufrieva K, Castillo R, Gao C, Liu J, Shahriari N, Hashemi K, Taylor D, Rowley R, Rainone E, LaChance A, Gate R, Korsunsky i, Vleugels R, Wei K. Modulation of Type I Interferon Signaling by Anifrolumab Alters the Spatial Immune Landscape in Cutaneous Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/modulation-of-type-i-interferon-signaling-by-anifrolumab-alters-the-spatial-immune-landscape-in-cutaneous-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/modulation-of-type-i-interferon-signaling-by-anifrolumab-alters-the-spatial-immune-landscape-in-cutaneous-lupus-erythematosus/