Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Ianalumab (VAY736) is an afucosylated monoclonal antibody targeting the B cell activating factor receptor (BAFFR) that depletes B cells via enhanced antibody dependent cellular cytotoxicity with concurrent blockade of BAFF:BAFFR mediated survival signals. BAFF is commonly overexpressed in systemic lupus erythematosus (SLE) and strongly linked to autoimmune pathogenesis. Here we assessed changes within the transcriptomic and proteomic landscape of SLE patients pre- and post-treatment with ianalumab to characterize pharmacodynamic (PD) biomarkers and biological pathways linked to the disease activity and clinical response.
Methods:
A phase 2 clinical trial (NCT03656562) evaluated the PD and preliminary clinical efficacy of ianalumab in patients with moderate to severe SLE; a multicenter randomized, parallel group, double blind, placebo controlled trial with patients on monthly s.c. injection of ianalumab 300 mg or placebo. Blood samples were collected at baseline and week (W) 28 post treatment from placebo (n=28) and ianalumab treated patients (n=25). Blood samples from 40 healthy volunteers were included for baseline transcriptomics analysis. Targeted cellular analysis was done by flow cytometry, serum protein profiles were generated using the SomaScan v4.1 assay and transcriptome analysis was done with total RNA obtained from whole blood using an Illumina Stranded kit. Treatment and placebo groups were compared over time using multivariate linear regression models to identify cellular markers, proteins and genes that were differentially modulated by ianalumab.
Results:
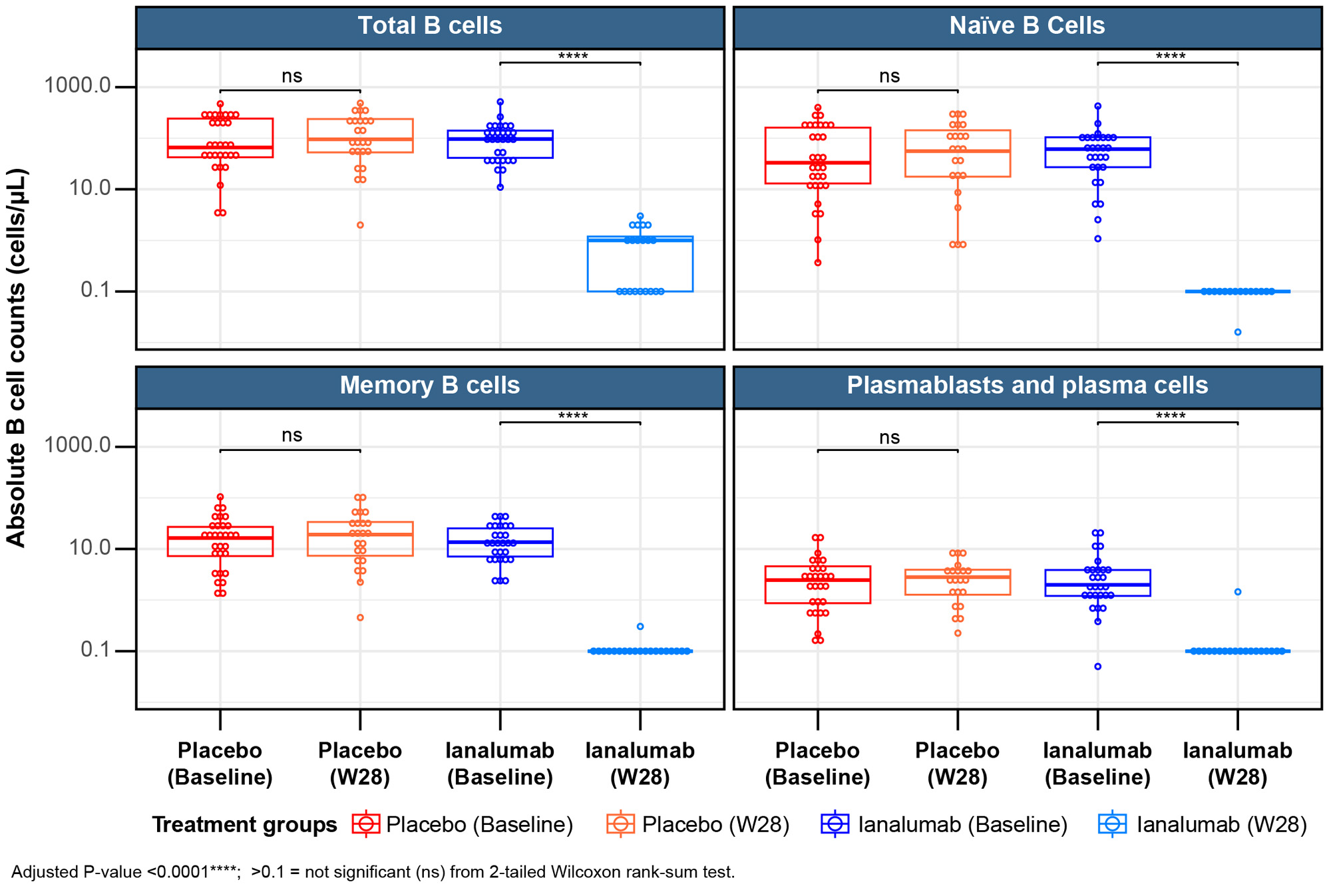
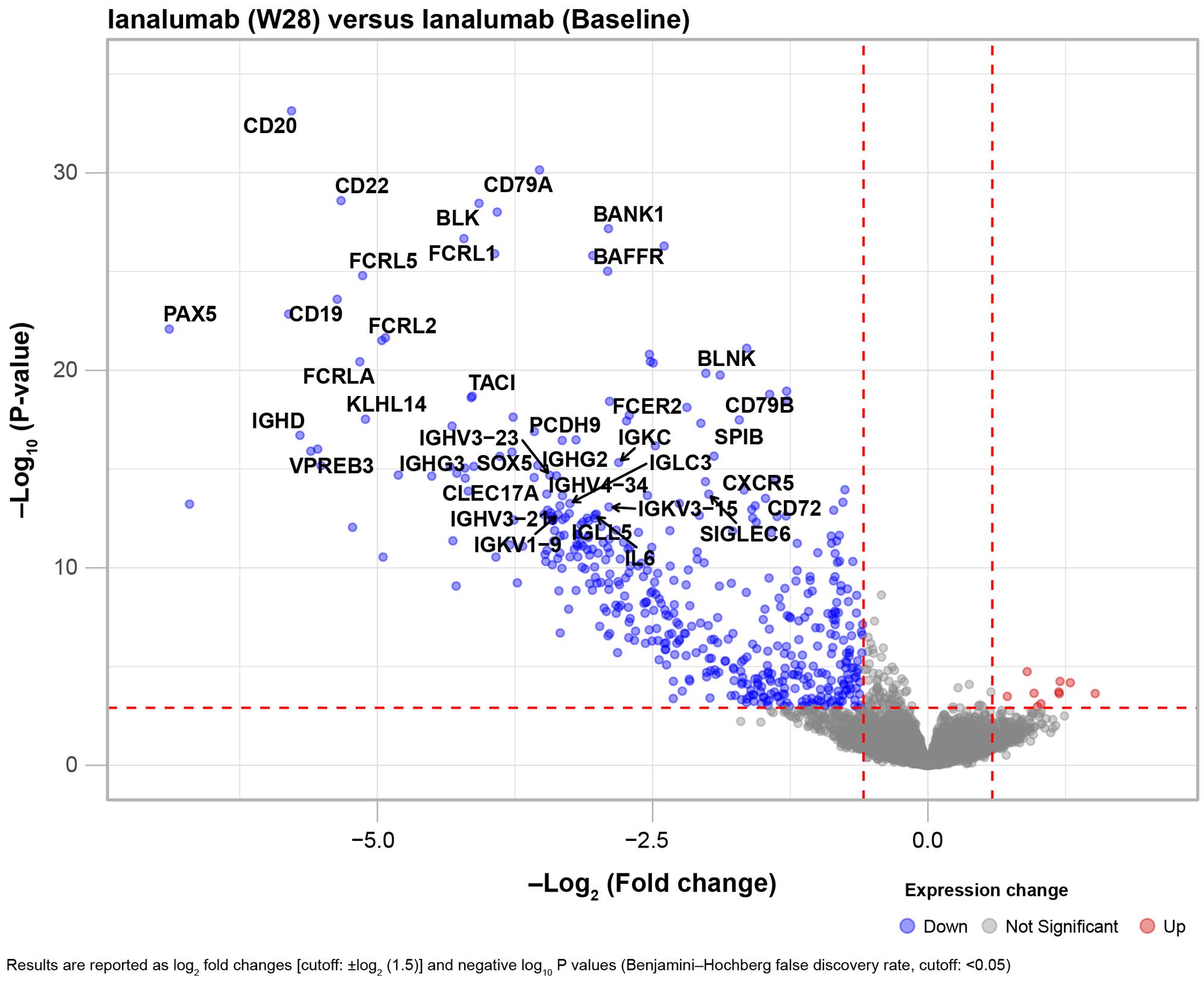
The analysis of changes to cellular subsets upon ianalumab treatment demonstrated a strong and statistically significant reduction in total B cells which was confirmed by changes in transitional, naïve and memory B cells as well as plasmablasts/plasma cells without reductions in CD4+ and CD8+ T cells or NK cells (Fig 1). Assessment of SLE molecular drivers through blood transcriptomics versus healthy volunteers confirmed a disease signature driven by type 1 interferon (IFN) and innate immunity pathways, correlating with immunoglobulin genes. Upon ianalumab treatment, a strong reduction in B cell related genes was seen at W28, accompanied by a significant decrease in IL-6 transcript and a modulation of the interferon gene signature (IFNGS) (Fig 2). Broad serum protein profiling confirmed transcriptomics findings, showing reduction of multiple B cell markers on ianalumab treatment. Importantly, we observed that SRI-4 response to ianalumab was comparable in both baseline IFNGS-high and IFNGS-low patients, with a concomitant SLEDAI reduction.
Conclusion:
Ianalumab treatment of patients with SLE led to strong B cell depletion, including autoantibody-producing subsets, confirmed by transcriptomics and proteomics assessments. Initial evidence suggests that reductions in B cell numbers and autoantibodies levels observed clinically was accompanied by a decrease in IFNGS and clinical response to ianalumab was independent of baseline IFNGS status. Further molecular studies are ongoing to characterize the effects of ianalumab on these patients.
To cite this abstract in AMA style:
Santos da Costa A, Dörner T, Grioni A, Avrameas A, Sommer U, Hillenbrand R, De Luca V, Ferrero E, Nogueira da Costa A, Isnardi I, J Oliver S. Modulation of B Cell and Interferon Pathways by Ianalumab in Patients with Systemic Lupus Erythematosus: Findings from a Phase 2 Clinical Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/modulation-of-b-cell-and-interferon-pathways-by-ianalumab-in-patients-with-systemic-lupus-erythematosus-findings-from-a-phase-2-clinical-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/modulation-of-b-cell-and-interferon-pathways-by-ianalumab-in-patients-with-systemic-lupus-erythematosus-findings-from-a-phase-2-clinical-trial/