Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Hyperinflammation is a life-threatening systemic inflammatory state most commonly associated with Hemophagocytic Lymphohistiocytosis (HLH) and Macrophage Activation Syndrome (MAS), but observed in nearly all inflammatory contexts. Clinical and mechanistic observations have identified many genetic and/or acquired factors that contribute to hyperinflammation, including profound cytotoxic impairment (e.g. perforin deficiency) and excess IL-18 among many others, all of which seem to converge on excessive cytotoxic T-lymphocyte (CTL) activation. CTL activation occurs at its physical interface with antigen-presenting target cells, known as the Immune Synpase (IS). To better understand how different HLH contributors, alone and in combinations, drive HLH, we sought to model these contributors through in vitro quantitation of CD8 T-cell IS characteristics
Methods: We performed live-cell microscopy of co-cultures of murine TCR-transgenic T-cells and various peptide-pulsed target cells in the presence of cell-impermeable viability dye. T-cells were loaded with Fluo-4 to enable detection of calcium flux. Co-culture supernatants were measured for cytokine accumulation by Cytometric Bead Array.
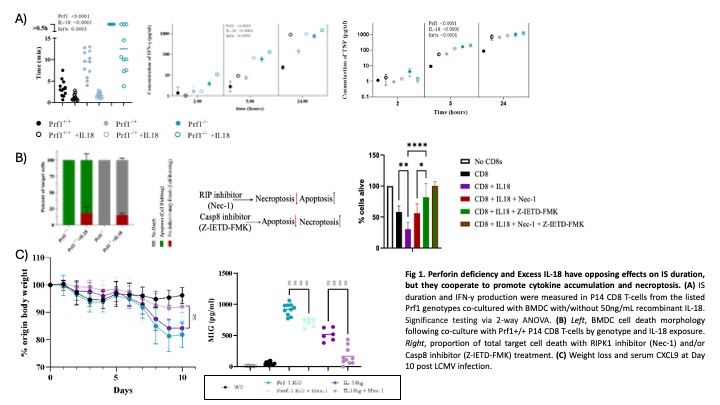
Results: We detected a gene-dose effect of Prf1 deficiency on IS duration and cytokine accumulation (Fig 1A). We observed similar results with cytotoxicity-normal T-cells by impairing IS termination using transformed target cells (fibrosarcoma cells or immortalized bone marrow derived macrophages). By contrast, weaker T-cell Receptor (TCR) signal strength led to IS prolongation but less cytokine accumulation. The addition of IL-18 to co-cultures decreased IS duration but increased IFNg accumulation and synergized with Prf1 haploinsufficiency in further driving cytokine accumulation (Fig 1A). Unexpectedly, exogenous IL-18 in co-cultures acted on T-cells to induce a small proportion of target cells to die by swelling rather than apoptotic contraction, even in co-cultures using Prf1-/- T-cells (Fig 1B). This IL-18 induced target cell swelling was inhibitable by RIPK1 (necroptosis) but not Caspase-8 (apoptosis) inhibition, suggesting necroptotic cell death. In vivo, necrostatin improved HLH/MAS symptoms in Il18tg mice more profoundly than Prf1-/- mice when infected with LCMV (Fig 1C).
Conclusion: Together, these results suggest that IS duration, T-cell cytokine production intensity, and mode of target cell death are interacting features of the IS that may independently contribute to the development of HLH-like immunophysiology. This in vivo platform, assessing these three IS characteristics, may be useful for assessing other potential HLH susceptibiliy factors, screening for new ones, and assessing the likelihood of cellular therapies to induce life-threatening hyperinflammation.
To cite this abstract in AMA style:
Frank-Kamenetskii A, Varghese J, Morrissette J, Klinghoffer H, Diorio C, Burkhardt J, Canna S. Modeling and Predicting HLH Through Measurement of Immune Synapse Duration, Cytokine Production, and Target Cell Death [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/modeling-and-predicting-hlh-through-measurement-of-immune-synapse-duration-cytokine-production-and-target-cell-death/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/modeling-and-predicting-hlh-through-measurement-of-immune-synapse-duration-cytokine-production-and-target-cell-death/