Session Information
Date: Wednesday, November 13, 2019
Title: 6W017: Health Services Research II: Health Economics (2888–2893)
Session Type: ACR/ARP Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: To assess the cost-effectiveness of various combinations of urate lowering therapy (ULT) and anti-inflammatory treatment in the management of newly diagnosed gout patients, from the Dutch societal perspective.
Methods: The Anakinra versus Treatment as usual in the Treatment of ACute Gout (ATTACG) study was the primary data source for this study. Since only patients with crystal proven gout were included in that study, all patient satisfy the ACR/EULAR gout classification criteria.
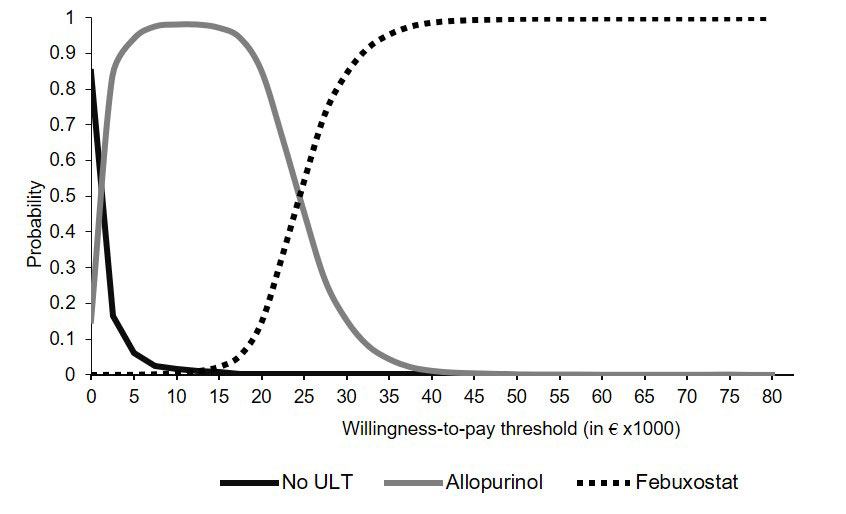
A probabilistic patient-level simulation estimating costs and quality-adjusted life years (QALYs) was performed to compare gout and hyperuricemia treatment strategies. Pain was the main determinant of both QALYs and costs, with disutility assigned to patients who failed to reach the serum urate (SUA) target (< 0.36 mmol/L). Patients faced a daily risk of a gout flare, with a higher risk for patients who failed to achieve the SUA target level. Health states were no flare, severe pain, mild pain, moderate pain, or no pain in the presence of a flare. Model input was derived from patient level clinical trial data (i.e. SF-6D utility, indirect costs in €’s and pain transition probabilities), meta-analyses or from previously published health-economic evaluations. The results of probabilistic sensitivity analyses were presented using incremental cost-effectiveness ratios (ICERs), and summarized using cost-effectiveness acceptability curves (CEAC). Scenario analyses were performed for gout patients not necessarily experiencing a gout flare at model entry, and for patients with a higher daily flare probability.
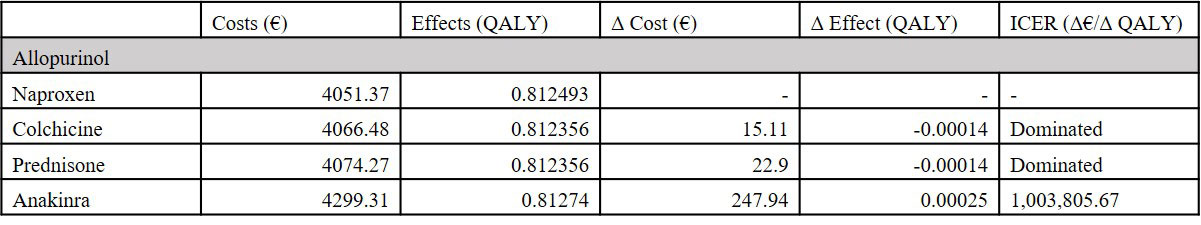
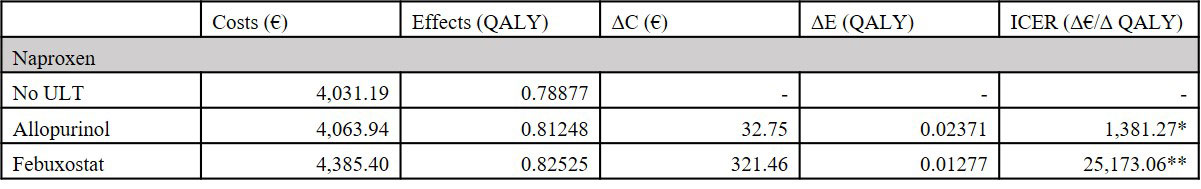
Results: In the base case, the ICER for allopurinol versus no ULT was €1,381, when combined with naproxen. Febuxostat yielded the highest utility but also the highest costs (€4,385 vs. €4,063 for allopurinol), resulting in an ICER of €25,173 when compared to allopurinol, all combined with naproxen. No ULT with naproxen was not cost-effective, yielding the lowest utility. For the gout flare medications, comparable effects on utility were achieved. Combined with allopurinol, naproxen was the cheapest option (€4,051), and anakinra the most expensive (€4,299). The ICER of anakinra compared to naproxen, when combined with allopurinol was €1,003,805.67 . Colchicine and prednisone were dominated by naproxen when combined with any of the ULT options. Results of the scenario analyses did not change the conclusions drawn from comparison of ULT and anti-inflammatory treatment. Results remained robust in scenario analyses.
Conclusion: Allopurinol and febuxostat were both cost-effective compared to No ULT. Febuxostat was cost-effective when compared to allopurinol in case of higher willingness-to-pay thresholds. Anakinra was not cost-effective at any regularly acceptable willingness-to-pay threshold. For treating acute gout flares, colchicine, naproxen and prednisone offered comparable health economic implications. However, naproxen was the favoured and dominant option.
To cite this abstract in AMA style:
van de Laar C, Oude Voshaar M, Janssen C, Janssen M, Al M, van de Laar M. Model-based Cost-Effectiveness Analyses Comparing Combinations of Urate Lowering Therapy and Anti-Inflammatory Treatment in Newly Diagnosed Gout Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/model-based-cost-effectiveness-analyses-comparing-combinations-of-urate-lowering-therapy-and-anti-inflammatory-treatment-in-newly-diagnosed-gout-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/model-based-cost-effectiveness-analyses-comparing-combinations-of-urate-lowering-therapy-and-anti-inflammatory-treatment-in-newly-diagnosed-gout-patients/