Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Fibromyalgia (FM) is among the most common chronic pain conditions, clinically characterized by widespread pain and fatigue. FM pathogenesis remains unknown, leading to challenges in diagnosis and treatment. Mitochondria are the headquarters of cellular energy metabolism and their malfunction has been proposed to contribute to both fibromyalgia and chronic fatigue. Thus, the aim of the current study was to detect structural changes in mitochondria of peripheral blood mononuclear cells (PBMCs) of FM patients, using transmission electron microscopy (TEM).
Methods: To detect mitochondrial structural alterations in FM patients, we analyzed PBMCs from seven FM patients and seven healthy controls using TEM. Patients were recruited from a specialized fibromyalgia clinic at a tertiary hospital. After providing informed consent, patients responded to questionnaires, including the Widespraede Pain Index (WPI) and the symptom severity Scale (SSS), to verify a diagnosis of FM according to ACR criteria. Subsequently, blood samples were drawn and PBMCs were collected for EM analysis.
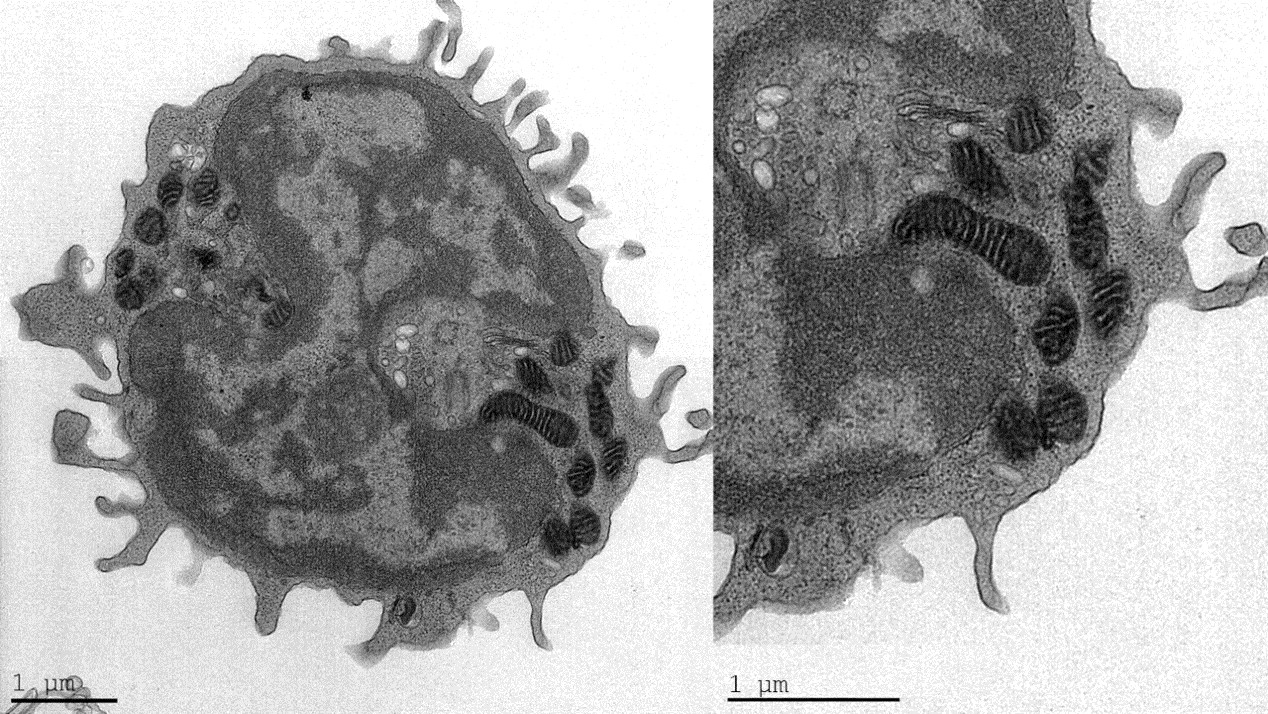
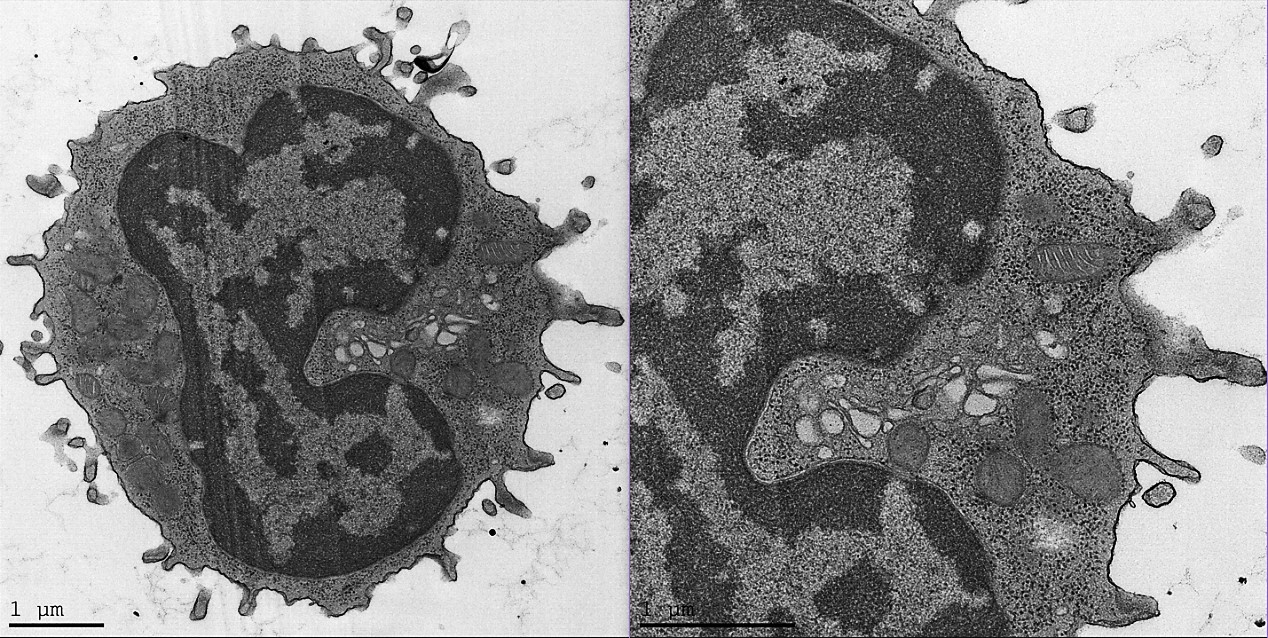
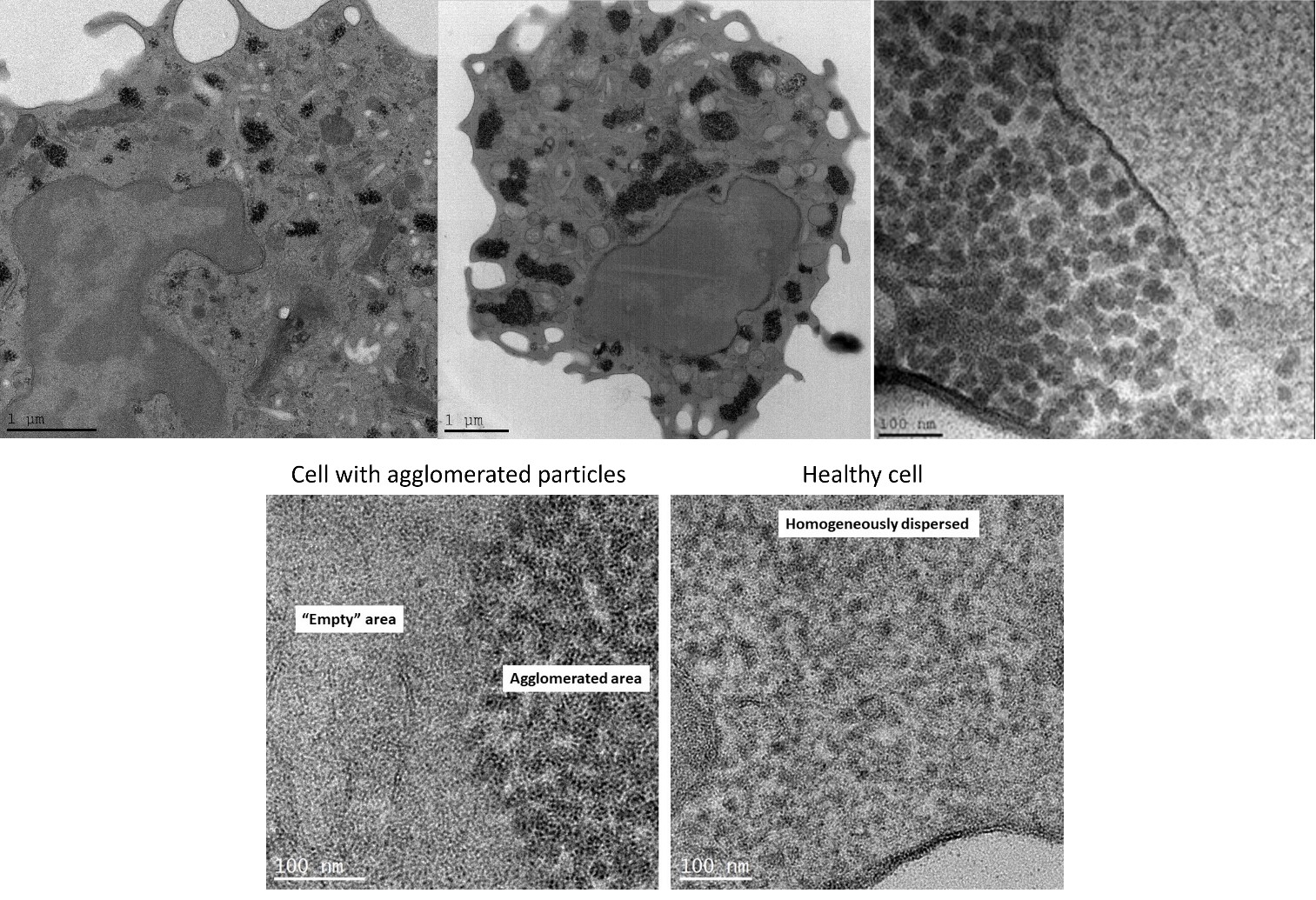
Results: TEM analysis of PBMCs showed several distinct mitochondrial patterns, including total loss of normal crista in FM patients (Fig. 1). The number of mitochondria with intact crista morphology was reduced in FM patients and the percentage of mitochondria that completely lacked crista was significantly increased. These results correlated with WPI severity. Moreover, in FM patient samples we observed a high percentage of cells containing “black-particles” (Fig. 2), which may represent ribosomal aggregates. Crista loss and possible ribosome aggregation were intercorrelated, and thus may represent reactions to a shared cellular stress condition. These changes may indicate an adaptive hypometabolic “hibernation-like” state to a stressful event, similar to hibernation in some mammalian species. The changes in mitochondria morphology suggest that mitochondrial dysfunction, resulting in inefficient respiration, Reactive Oxygen Species generation, etc., may play a pathogenetic role in FM.
Conclusion: We here describe novel morphological changes in mitochondria of FM patients, including loss of mitochondrial crista. While these observations cannot determine whether the changes are pathogenetically primary, or represent an epiphenomenon, they highlight the possibility that mitochondrial malfunction may play a causative role in the cascade of events leading to chronic pain and fatigue in FM. Moreover, the results offer the possibility of utilizing changes in mitochondrial morphology as an objective biomarker in FM. Further understanding the connection between FM and mitochondria morphology, function, and turnover (biogenesis/mitophagy), may assist in developing both novel diagnostic tools as well as specific treatments for FM.
To cite this abstract in AMA style:
Israel L, Furer V, Gross A, Ablin J. Mitochondrial Structural Alterations in Fibromyalgia – A Pilot Electron Microscopy Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/mitochondrial-structural-alterations-in-fibromyalgia-a-pilot-electron-microscopy-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mitochondrial-structural-alterations-in-fibromyalgia-a-pilot-electron-microscopy-study/