Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Hydroxychloroquine (HCQ) prolongs disease-free and damage-free survival in lupus (SLE). Yet, ~80% of patients stop taking HCQ resulting in poor outcomes including early mortality. High nonadherence could be linked to complexity in treatment decisions and lack of support in decision-making. Unable to weigh benefits vs. rare harms of HCQ and align treatment goals with values, patients have greater risk aversion leading to nonadherence. Shared decision-making (SDM) tools can support patient-clinician communication, align treatment goals with values, and motivate patient participation in decision-making. Thus, we aimed to test the efficacy of an SDM tool (HCQ-SAFE©) in a) improving HCQ adherence; b) resolving decisional conflicts around HCQ in SLE.
Methods: HCQ-SAFE© (hcqsafe.medicine.wisc.edu) is a 5-domain pictorial e- and paper-tool highlighting key benefits vs. rare harms of HCQ, developed by our group in English & Spanish using a collaborative iterative process involving 30 advisors (Garg et al. 2023). To test the efficacy of HCQ-SAFE©, we recruited 200 adults with SLE on HCQ across 8 clinics in 2 diverse health systems (NCT05922722). The primary outcome was HCQ adherence measured using proportion of days covered (PDC) 3-6 months before and after HCQ-SAFE© review; nonadherence defined as PDC < 80%. The secondary outcome was decision conflict scale (DCS) scores measured pre- and post-intervention using the low literacy 10-item DCS questionnaire (DCS scores range: 0 (best) - 100 (worst); ≥25 indicate high decisional conflicts). We used paired t-test and adjusted linear regression analyses to examine change in 1) HCQ adherence; 2) DCS scores pre- vs. post-HCQ-SAFE© review.
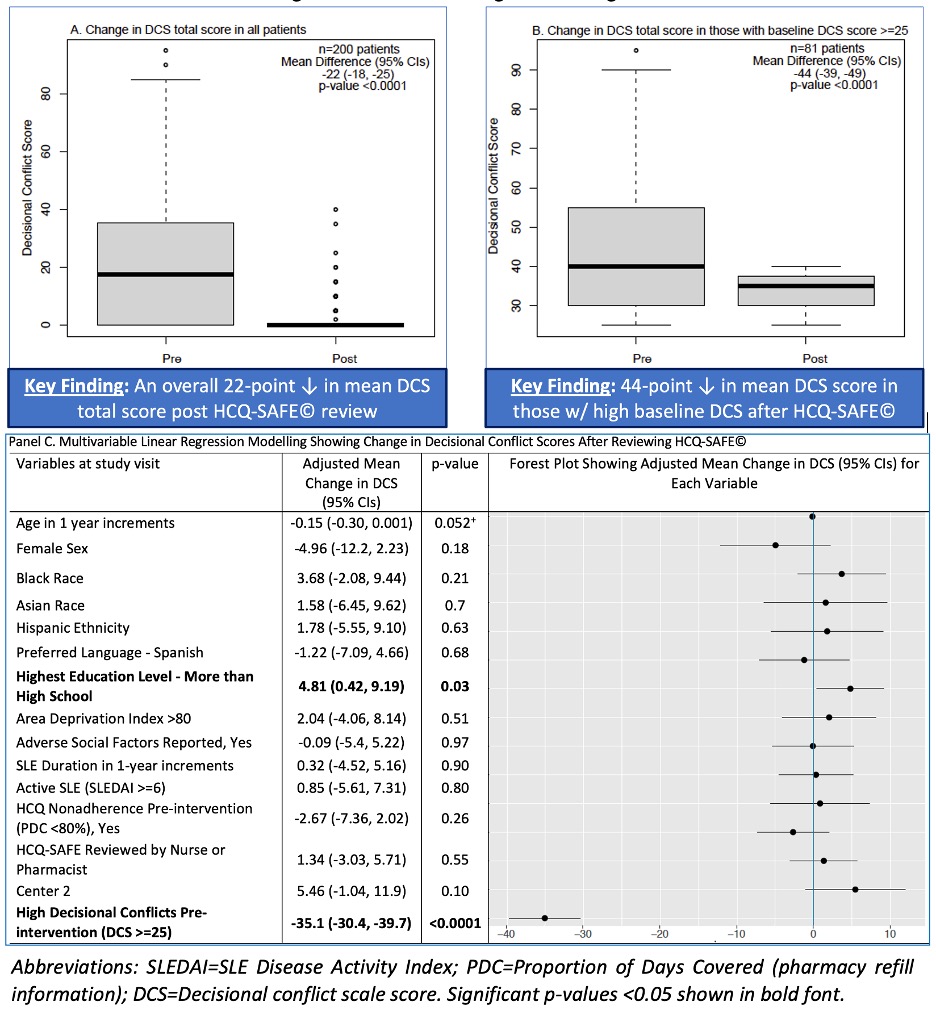
Results: Among 200 participants, 90% were women, 10% preferred Spanish, 54% had high decisional conflicts at baseline. SDM during visits was completed by MDs (50%), RNs (21%), and pharmacists (29%). Mean±SD time spent to review HCQ-SAFE© was 6±2 mins. Intervention completion rate was 100%. Next, a significant increase in HCQ adherence (post-PDC) by 47% was noted after HCQ-SAFE© review compared to baseline HCQ adherence (pre-PDC) after adjusting (Table. 1). Using pre-PDC adherence categories of 0%, < 40%, 40-79%, ≥80% (Hsu et al, 2018), an increase in post-PDC adherence by 29%, 56%, and 81% was noted in 0%, < 40%, 40-79% categories vs. ≥80% category (reference) after HCQ-SAFE© review (Table 2).After reviewing HCQ-SAFE©, a 22-point and a 44-point reduction in DCS scores was seen in all patients (p < 0.001; Fig 1A), and those with high DCS (≥25) at baseline (Fig. 1B), respectively. After adjusting, a 35-point reduction in DCS was noted post-intervention in patients with high DCS at baseline (Fig 1C). In analysis stratified by language, clinician, and education level, similar significant reductions were seen in DCS scores and PDC after HCQ-SAFE© review (p < 0.0001, data not shown).
Conclusion: HCQ-SAFE© provides clear, concise information, reducing decisional conflicts and significantly improving adherence across SLE populations. SDM tools like HCQ-SAFE© can mitigate nonadherence due to limited health literacy, linguistic clarity, lack of decision support, potentially improving SLE outcomes.
Panel A. Decisional conflict scale (DCS) scores pre- and post-intervention (HCQ-SAFE© review) in all patients across 8 clinics and 2 health centers (n=200);
Panel B. Decisional conflict scale (DCS) scores pre- and post-HCQ-SAFE© review in patients with high DCS scores at baseline (pre-intervention) defined as DCS ≥25;
Panel C. A multivariable linear regression model showing mean change in DCS after HCQ-SAFE© review.
.jpg) Table 1. A Multivariable Linear Regression Model Showing Change in HCQ Adherence (Proportion of Days Covered (PDC)) After HCQ-SAFE© Review
Table 1. A Multivariable Linear Regression Model Showing Change in HCQ Adherence (Proportion of Days Covered (PDC)) After HCQ-SAFE© Review
.jpg) Table 2. A Multivariable Linear Regression Model Showing Change in HCQ Adherence After HCQ-SAFE© Review Using pre-PDC adherence categories of 0%, < 40%, 40-79%, ≥80% (Hsu et al, Rheumatology Oxford, 2018)
Table 2. A Multivariable Linear Regression Model Showing Change in HCQ Adherence After HCQ-SAFE© Review Using pre-PDC adherence categories of 0%, < 40%, 40-79%, ≥80% (Hsu et al, Rheumatology Oxford, 2018)
To cite this abstract in AMA style:
Hartel I, Gazeley D, Patel J, Chewning B, Gomez S, Michaud J, Dickmann L, keevil J, Tellez-giron P, Bartels C, Garg S. Mitigating Hydroxychloroquine (HCQ) Nonadherence by Clarifying Misbeliefs Using a Shared Decision-Making Tool (HCQ-SAFE©) Across Two Different Rheumatology Centers [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/mitigating-hydroxychloroquine-hcq-nonadherence-by-clarifying-misbeliefs-using-a-shared-decision-making-tool-hcq-safe-across-two-different-rheumatology-centers/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mitigating-hydroxychloroquine-hcq-nonadherence-by-clarifying-misbeliefs-using-a-shared-decision-making-tool-hcq-safe-across-two-different-rheumatology-centers/