Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: The effect of bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)‑17F in addition to IL-17A, on structural radiographic progression in the spine has not yet been reported in patients (pts) with radiographic axial spondyloarthritis (r-axSpA). Here, we report the impact of BKZ on 2-year spinal radiographic progression and new syndesmophyte formation in pts with r-axSpA in the open-label extension (OLE) of the phase 3 BE MOBILE 2 study.
Methods: The BE MOBILE 2 (NCT03928743) study design has been reported previously.1 At Week (Wk) 52, eligible pts with r-axSpA could enroll in an ongoing OLE (NCT04436640) and continue BKZ. Baseline (BL) and Wk 104 spinal radiographs were assessed using modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) by 2 central readers; change scores differing by ≥5 points were adjudicated. All readers were blinded to timepoint. The average score change across readers was determined for each radiograph; if 3 readers were used, the average of the 2 closest change scores was calculated. Syndesmophytes were recorded if identified by 2 reviewers at a given anatomical site. New syndesmophytes were defined as syndesmophytes declared present at Wk 104 but not at BL at the same site.
For pts with mSASSS available at BL and Wk 104, we report mean and cumulative probability of change from baseline (CfB) in mSASSS at Wk 104, and the proportion of non‑progressors, using definitions mSASSS CfB ≤0.5 and mSASSS CfB < 2. We also report the proportion of pts with new syndesmophytes at Wk 104 in those with/without BL syndesmophytes. Potential predictive factors for spinal radiographic progression (mSASSS CfB ≥2) at Wk 104 were assessed using logistic regression models.
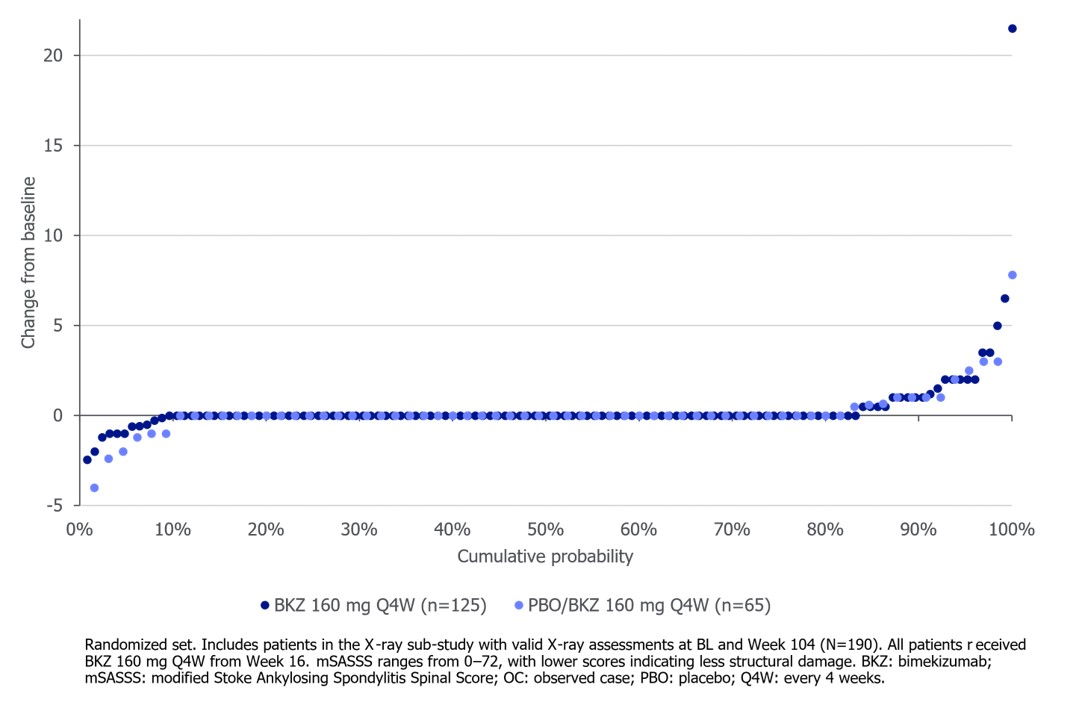
Results: Of 332 randomized pts, 286 (86.1%) entered the OLE; 267 (80.4%) completed Wk 104. Of those who completed Wk 104, 71.2% (190/267) of pts had an mSASSS available at BL and Wk 104. Mean (SD) BL mSASSS was 7.3 (13.8), with a CfB of 0.3 (1.9) at Wk 104. The majority of pts (157/190; 82.6%) had no spinal radiographic progression at Wk 104 (mSASSS CfB ≤0; Figure).
The proportion of non‑progressors at Wk 104 (mSASSS CfB ≤0.5) was 85.3% (162/190). When defined as mSASSS CfB < 2, 92.1% (175/190) were non-progressors, including 83.1% (69/83) of pts with existing BL structural damage (mSASSS ≥2).
BL syndesmophytes were present in 57/190 pts (30.0%). At Wk 104, only 12/57 pts (21.1%) with BL syndesmophytes and 2/133 pts (1.5%) without BL syndesmophytes had developed new syndesmophytes.
Of the potential predictive factors assessed using the univariable models, presence of BL syndesmophytes, non-White ethnicity, and positive HLA-B27 were found to significantly increase likelihood of spinal radiographic progression (mSASSS CfB ≥2) at Wk 104.
Conclusion: Minimal spinal radiographic progression was demonstrated at 2 years of BKZ treatment in pts with r-axSpA. A high proportion of pts were non-progressors, including in those with BL spinal damage. A minor fraction of pts developed new syndesmophytes at 2 years of BKZ treatment, including almost one fifth of pts with existing BL syndesmophytes.
References: 1. van der Heijde D. Ann Rheum Dis 2023;82(4):515–26.
To cite this abstract in AMA style:
Baraliakos X, Ramiro S, Maksymowych W, Ostergaard M, Massow U, Vaux T, Prajapati C, Marten A, de Peyrecave N, Poddubnyy D. Minimal Spinal Radiographic Progression in Patients with Radiographic Axial Spondyloarthritis over 2 Years of Bimekizumab Treatment: Results from a Phase 3 Open-Label Extension Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/minimal-spinal-radiographic-progression-in-patients-with-radiographic-axial-spondyloarthritis-over-2-years-of-bimekizumab-treatment-results-from-a-phase-3-open-label-extension-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/minimal-spinal-radiographic-progression-in-patients-with-radiographic-axial-spondyloarthritis-over-2-years-of-bimekizumab-treatment-results-from-a-phase-3-open-label-extension-study/