Session Information
Date: Sunday, November 13, 2022
Title: Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Though continuous composite measures of disease activity for psoriatic arthritis (PsA) assessment exist, abbreviated measures that are more feasible for screening in routine clinical practice are needed. The 3 Visual Analogue Scale (VAS) and 4 VAS scores were developed by abridging the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Composite Exercise (GRACE) measure to be the first short multidimensional composite measures specifically for use in PsA routine clinical care. The measures were shown to have superior performance than several established composite measures using small datasets,1. GRAPPA members recommended further testing of 3VAS/4VAS in observational and trial datasets.
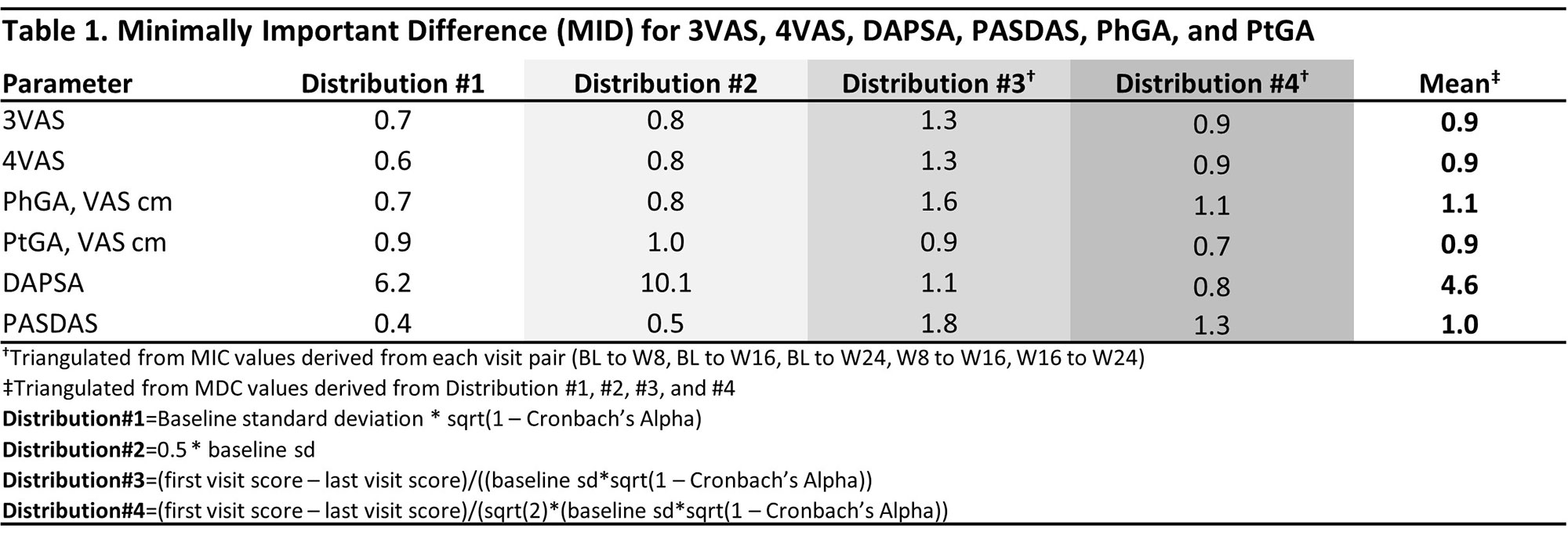
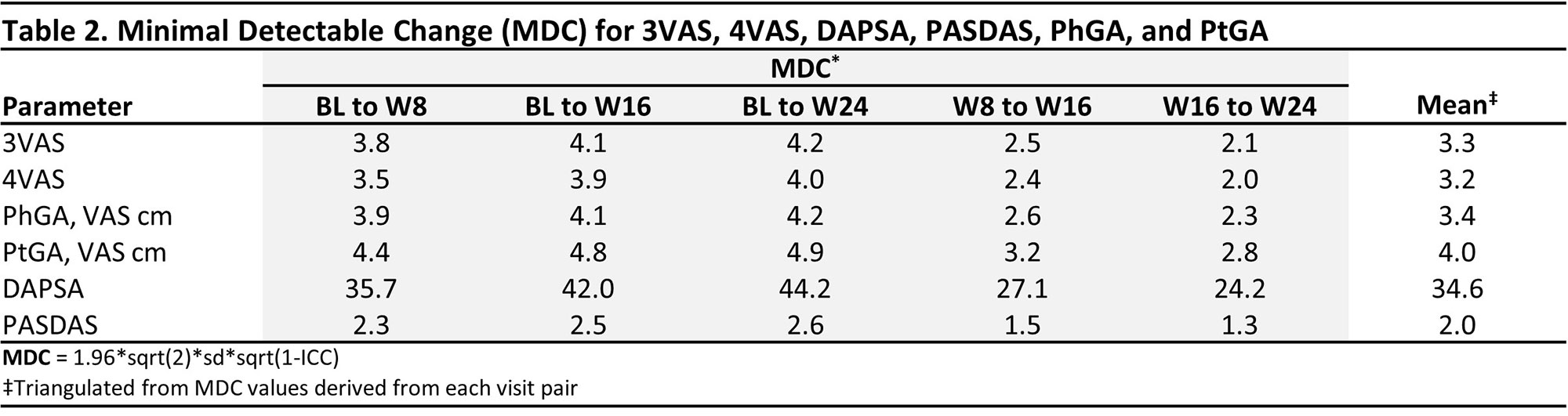
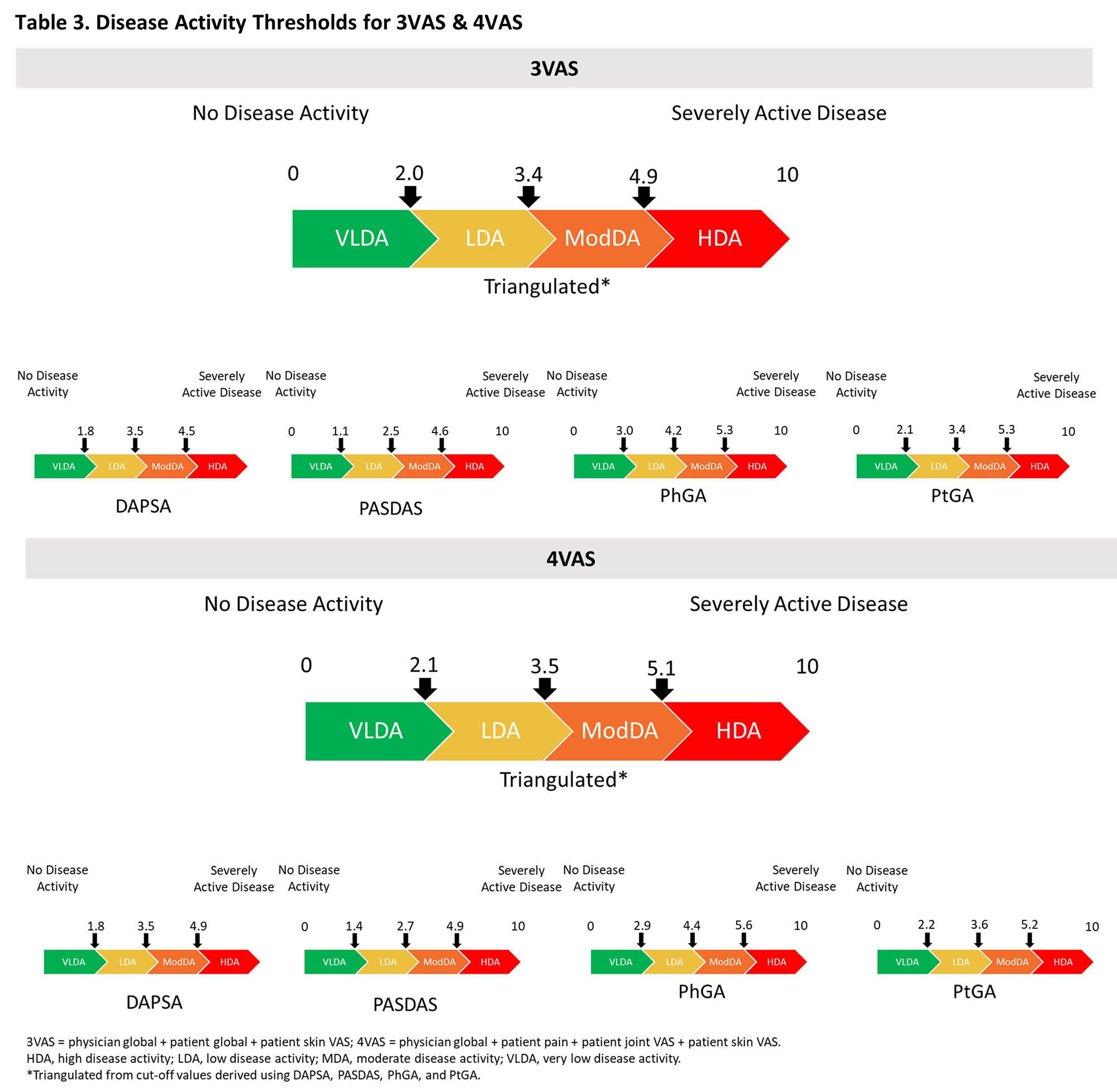
Methods: This post-hoc analysis of the DISCOVER 1/2 and COSMOS studies2-4 used pooled data through week (W) 24 from all treatment groups across studies. The correlation of 3VAS/4VAS with DAPSA, PASDAS, physician global assessment (PhGA), and patient GA (PtGA) was assessed with Pearson’s correlation coefficient. Minimal Important Difference (MID) was assessed with 4 distribution-based methods (based on standard error of the measurement, effect size, reliable change index [RCI], and RCIdiff). Minimal detectable change (MDC) was assessed with the standard formula (Table 2). Clinically relevant thresholds for low, moderate and high disease activity were estimated with receiver operating characteristic analysis and DAPSA (≤4, >4-≤14, >14-≤28, >28), PASDAS (≤1.9, >1.9-≤3.2, >3.2-< 5.4, ≥5.4), and PhGA/PtGA (≤1, >1-≤3, >3-≤6, >6 cm) as anchors.
Results: 1,405 pts were included, of whom 51.3% were male, with a mean (SD) age of 47.1 (11.8), and PsA duration of 6.4 (6.5) years. At BL, the mean (SD) 3VAS, 4VAS, DAPSA, PASDAS, PhGA, and PtGA scores of 6.4 (1.6), 6.3 (1.6), 45.8 (20.2), 6.5 (1.1), 6.5 (1.6), and 6.7 (2.0), respectively, reflected high levels of disease activity. Through W24, both 3VAS and 4VAS showed very strong correlation with PtGA (r3VAS=0.92, r4VAS=0.94) and PASDAS (r3VAS=0.81, r4VAS=0.82), strong with PhGA (r3VAS=0.77, r4VAS=0.74), and moderate to strong with DAPSA (r3VAS=0.59, r4VAS=0.61). Calculated MIDs were 0.9 (range: 0.7-1.3 across methodologies) for 3VAS and 0.9 (range: 0.6-1.3) for 4VAS (Table 1). MDC estimates were 3.3 (range 2.1-4.2 across follow-up intervals) for 3VAS and 3.2 (range: 2-4) for 4VAS (Table 2). Cut-off values for low, moderate and high disease activity were 2.0, 3.4, and 4.9 for 3VAS and 2.1, 3.5 and 5.1 for 4VAS (Figure).
Conclusion: Using a large pooled clinical trial dataset of pts with active PsA, we have calculated clinically relevant thresholds for improvement, as well as disease activity thresholds, for 3VAS and 4VAS. These estimates are generally comparable to those previously reported5 and can be used to set treatment targets as well as screen disease activity in routine care when resources are limited or during remote pt monitoring.
References
To cite this abstract in AMA style:
Tillett W, Coates L, Vis M, Merola J, Soriano E, Perate M, Shawi M, Zimmermann M, Rampakakis E, Sharaf M, Nash P, Helliwell P. Minimal Important Difference (MID), Minimal Detectable Change (MDC), and Disease Activity Thresholds for Two Novel Composite Instruments (3VAS, 4VAS) in Patients with Psoriatic Arthritis: Pooled Analysis of Three Phase 3 Studies [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/minimal-important-difference-mid-minimal-detectable-change-mdc-and-disease-activity-thresholds-for-two-novel-composite-instruments-3vas-4vas-in-patients-with-psoriatic-arthritis-pooled-analy/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/minimal-important-difference-mid-minimal-detectable-change-mdc-and-disease-activity-thresholds-for-two-novel-composite-instruments-3vas-4vas-in-patients-with-psoriatic-arthritis-pooled-analy/