Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with Systemic lupus erythematosus (SLE) have significant impairment in quality of life (QOL). Health related QOL (HRQOL) is one of the four established core outcomes in SLE. Generic PRO instruments such as Short Form 36(SF-36), SF-6D, EuroQOL (EQ-5D) and the Functional Assessment of Chronic Illness Therapy (FACIT-Fatigue)) have been shown to be valid and reliable in SLE. The purpose of this study is to determine minimal clinically important difference (MCID) of SF-36 and FACIT-fatigue against varied physician assessed DA indices, using data sharing resource from Glaxo-Smith-Kline (GSK), collected during Belimumab clinical trial – BLISS 52.
Methods: Longitudinal data from BLISS 52 clinical trial from 867 SLE patients was acquired from GSK for purpose of these analyses. DA measures (SLEDAI, BILAG) were used as anchors. MCID estimates were obtained against all possible predefined DA and composite DA endpoints used in SLE clinical trials. Mixed model analysis was utilized for the first 4 visits, one month apart for these analyses for individual DA anchors. For SELENA-SLEDAI Flare Index (SFI) and composite measures, 6,069 observation data from 7 visits were used.
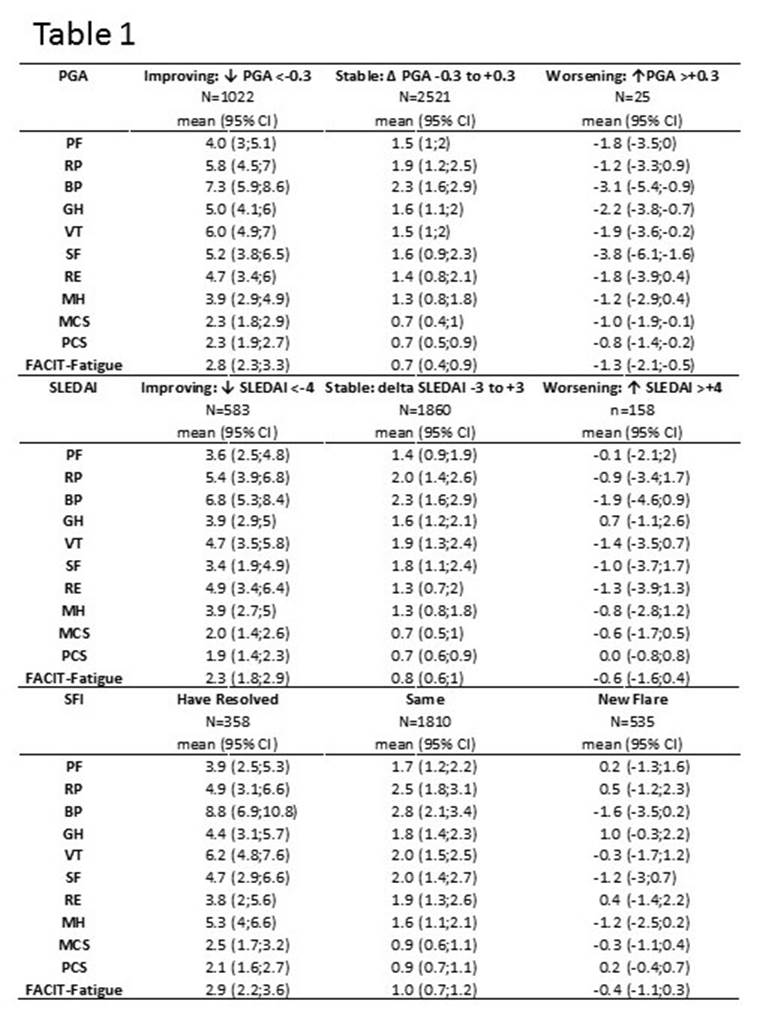
Results: 867 SLE patient data consisting of 95% women, with mean (SD) age 35.5 (11.1) yrs., were available. Nearly 65% were Asian or White ethnicity. Median (IQR) SLEDAI, BILAG were 10.0 (4.0) and 17 (11.0) respectively. Median (IQR) MCS, PCS, FACIT-Fatigue were 40.3 (14.3), 41.7 (12.9) and 34.0 (14.0) respectively. MCID against SLEDAI and SRI are shown in table 1 and table 2 respectively. Results for MCID against BILAG are not included in the table but will be available for presentation. SF36 domains and FACIT-fatigue were responsive to improvement in DA endpoints – SELENA-SLEDAI, SFI, BILAG and composite measures. SF36 domains and FACIT-Fatigue were not consistently responsive to worsening in DA.
Conclusion: Our study provides SLE specific MCIDs against commonly used DA measures and further reinforce the need for use of disease specific MCID over “generic” values of improvement/worsening by 2.5 and 5.0 in domain and composite scores of SF36.
 |
 |
To cite this abstract in AMA style:
Devilliers H, Annapureddy N, Jolly M. Minimal Clinically Important Differences for Generic Patient Reported Outcomes Tools in SLE [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/minimal-clinically-important-differences-for-generic-patient-reported-outcomes-tools-in-sle/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/minimal-clinically-important-differences-for-generic-patient-reported-outcomes-tools-in-sle/
