Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Type Ⅰ interferons (IFN) contribute to antiviral innate immune responses. Upon viral infection, pattern recognition receptors trigger TANK-binding kinase 1 (TBK1) activation and lead to activation of the transcription factor IFN regulatory factor (IRF) 3 to induce type Ⅰ IFN production. Because high expression of typeⅠ IFN inducible genes, called IFN signature, is observed in systemic lupus erythematosus (SLE), the excessive IFN production has been thought to have the important role in SLE pathogenesis.
Recently, it has been reported that silica acid-binding immunoglobulin-like lectin (Siglec) 1 suppresses antiviral innate immune response by inducing TBK1 degradation by the E3 ubiquitin ligase, tripartite motif-containing protein (TRIM) 27. It has also been reported that type Ⅰ IFN-induced downregulation of microRNA (miR)-27a can increase TRIM27 expression, and then inhibit the type Ⅰ IFN production in antiviral innate response.
Here, we report that downregulation of TRIM27 by miR-27a can contribute to IFN signature in SLE.
Methods: Human peripheral blood mononuclear cells (PBMC) and sera were obtained from SLE patients and healthy controls (HC). All of the patients fulfilled the revised 1997 ACR Criteria for Classification of SLE. The quantitative real-time PCR (qPCR) and western blotting were performed to assess the mRNA and protein expression levels of TRIM27 and TBK1, respectively. Serum miR-27a levels were investigated by qPCR. Statistical analysis was performed by using the GraphPad Prism. Data are presented as mean ± SEM. We used a Student’s t-test or the two-tailed Mann-Whitney U test. The p-values < 0.05 were considered statistically significant.
Results: The expression level of miR-27a in sera of SLE patients was higher than that of HC (p=0.0043; Figure 1A). The expression levels of mRNA and protein of TRIM27 in PBMC of SLE patients were lower than those of HC (p=0.020, p=0.038, respectively; Figure 1B, C). On the contrary, TBK1 protein level was higher in SLE patients as compared to HC (p=0.0079; Figure 2A, B).
Conclusion: In SLE patients, increased miR-27a expression may induce downregulation of TRIM27, which leads to reduction of the TBK1 ubiquitination and degradation. The results suggest that miR-27a can contribute to interferon signatures in SLE via the suppression of TRIM27.
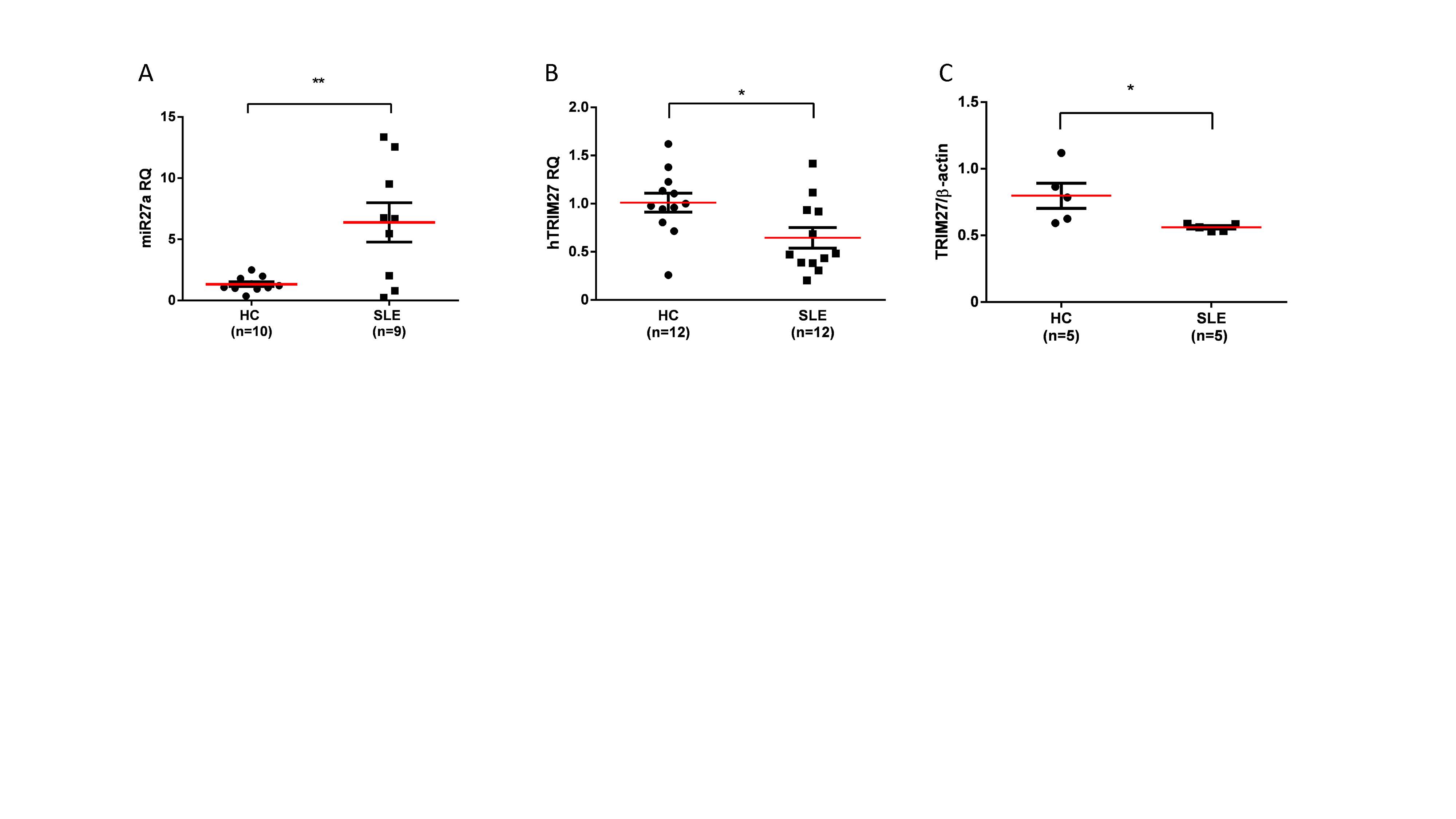
ACR2019_figure1 for abstract_Kishimoto_final
-A-The expression level of miR-27a in sera of SLE patients was higher than that of HC -p=0.0043-. The expression levels of mRNA -B- and protein -C- of TRIM27 in PBMC of SLE patients were lower than those of HC -p=0.020, p=0.038, respectively-. Data are presented as mean ± SEM. Statistically significant data -*, p< 0.05 and **, p < 0.01- by Student’s t-test or two-tailed Mann-Whitney U test.

ACR2019_figure2 for abstract_Kishimoto_final
-A- Western blot analysis was performed with extracts from PBMC of SLE patients and HC using anti-TBK1 Ab. β-actin was used as a loading control. -B- TBK1 protein level was higher in SLE patients as compared to HC -n=5 in each groups-. Data are presented as mean ± SEM. Statistically significant data -**, p < 0.01- by Student’s t-test.
To cite this abstract in AMA style:
Kishimoto D, Yoshimi R, Kunishita Y, Sugiyama Y, Komiya T, Sakurai N, Kamiyama R, Kirino Y, Nakajima H. MicroRNA-27a Can Contribute to Interferon Signatures in Systemic Lupus Erythematosus via the Suppression of Tripartite Motif-containing Protein 27 [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/microrna-27a-can-contribute-to-interferon-signatures-in-systemic-lupus-erythematosus-via-the-suppression-of-tripartite-motif-containing-protein-27/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/microrna-27a-can-contribute-to-interferon-signatures-in-systemic-lupus-erythematosus-via-the-suppression-of-tripartite-motif-containing-protein-27/
