Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Optimal dosing of low-dose methotrexate (MTX) is a challenge in rheumatoid arthritis (RA) because of substantial interpatient variation of response and because no stable plasma MTX levels are reached. MTX-polyglutamates (PG) in red blood cells (RBC) have been suggested as a potential marker of response, however contradictory results have been reported.
We aimed to evaluate the association between MTX-PG3-5 exposure and response in rheumatoid arthritis patients starting MTX and to identify clinically relevant covariates that could explain the inter-patient variability in this association.
Methods: Data of three prospective cohorts (MTX-R, tREACH and MeMo, The Netherlands, 395 patients) were available. RBC MTX-PG concentrations were determined by LC-MS/MS. Individual MTX-PG concentrations were summed in two categories: MTX-PG1-2 and MTX-PG3-5. The relationship between exposure and DAS28 was analysed using a semi-mechanistic pharmacokinetic-pharmacodynamic (PK-PD) model. In the population model relevant covariates were tested using full covariate modelling, followed by backward elimination. Reference values were adopted from Pan et al. 2014. Forest plots were used to define clinically significant covariates on MTX exposure and response. Covariates were considered clinically relevant if the 95% confidence interval (CI) of this covariate effect at the 1st and 9th quantile of the full dataset fell outside -25 or +25% deviation from the typical value. Only statistical significant covariates (p< 0.001) are displayed.
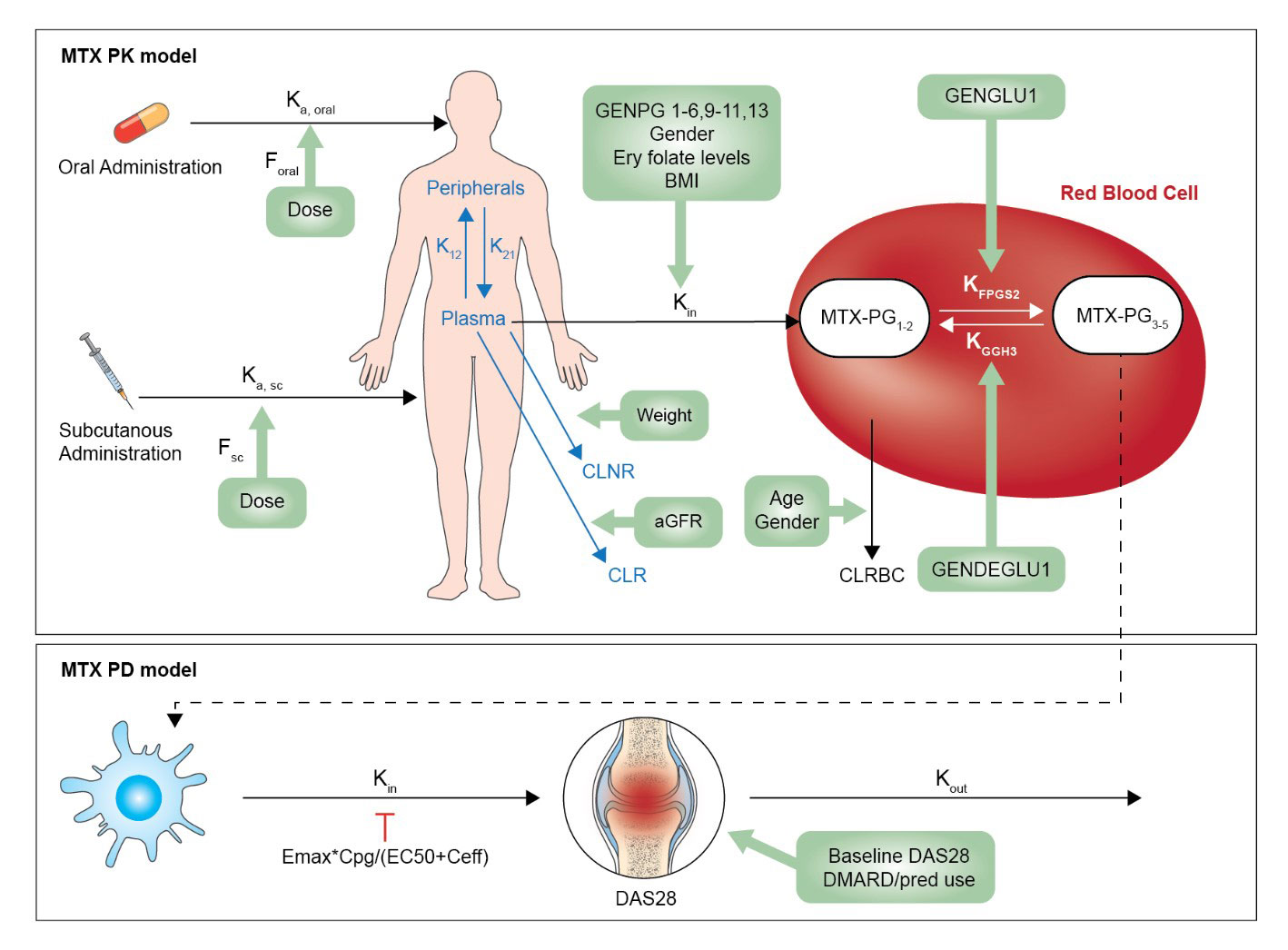
Results: We developed a PK-PD model (Fig 1) with 3387 individual MTX-PG concentrations and 1337 DAS28 outcome measurements between 0-300 days after the start of MTX treatment. The developed model adequately described the time course of MTX-PG3-5 and DAS28. The median MTX-PG3-5 level at month 1 was 30.9 nM (IQR 17.4) and at month 3: 69.3nM (IQR 56.9). Clearance of MTX-PG3-5 from RBCs was significantly lower (14%, 95%CI 8% – 17%) in females and older patients (log age=140% (CI 119%-164%, e.g. -38% (CI 32%-44%) for patients of 85 years compared with patients of 65 years). Patients with FPGS rs4451422 exhibited a reduced formation of MTX-PG345 (KFPGS2, 85%, CI 78%-93%). MTX-PG3-5 levels were associated with DAS28: Emax was 2.0 (95%CI 1.9-2.3) and an EC50 was 11.6 nM (CI 6.8nM-19.7nM), which is below most observed MTX-PG3-5 concentrations. Patients with a high baseline DAS28 showed a higher chance to improve (log DAS on EMAX: 153%, (CI 141%-166%), e.g. a baseline DAS28 of 5.0 vs 3.5 resulted in 55% (CI 50%-59%) higher EMAX). Co-administration of DMARDs and prednisolone lowered the EC50; 69% (CI 61%-79%) and 46% (CI 33%-66%), respectively. Smoking and BMI negatively influenced EMAX (not clinically relevant). None of the genetic variables influenced MTX-response.
Conclusion: With this PKPD model in RA patients starting methotrexate, exposure of methotrexate polyglutamates 3-5 in RBCs was associated with response. Baseline DAS28 and comedication affected the response clinically relevant. Low MTX-PG3-5 EC50 in RBCs was predictive for response, suggesting that the early measurements of long chain methotrexate polyglumates, before steady state is reached, are useful for dose individualisation.
PK pharmacokinetic, PD pharmacodynamic, Ka, oral absorption rate constant after oral absorption, Ka, sc absorption rate after subcutaneous absorption, DAS28 disease activity score measurement, K12 distribution rate constant of MTX-PG1 from plasma to peripheral tissue, K21 distribution rate constant of MTX-PG1 from peripheral tissue to plasma, Kin uptake rate constant of MTX-PG1 in RBC, CLRBC Clearance of MTX-PGs from RBCs, CLNR non-renal clearance, CLR Renal clearance, KFPGS2 polyglutamation rate constant from MTX-PG12 to MTX-PG345 KGGH3 deglutamation rate constant from MTX-PG345 to MTX-PG21, F bioavailability, MTX-PG methotrexate polyglutamate. GENPG1-6, 9_11, 13: rs1051266SLC19A1, rs2239907SLC46A1, rs13120400ABCG2, rs35592ABCC1, rs3784862ABCC1, rs3785911ABCC3, rs4793665ABCC3, rs868853ABCC4, rs2139560ABCC5. GENPD1-7: rs1801131MTHFR, rs1801133MTHFR, rs1801394MTRR, rs2372536ATIC, rs17602729AMPD1, rs1127354ITPA, rs5751876ADORA2A. GENDEGLU1: rs3758149GGH. GENGLU1: rs4451422FPGS. For all SNPs: 0=wildtype, 1=homozygous or variable. Emax: Maximum effect, Cpg: MTX-PG 3-5 concentration, EC50: half maximum effect concentration.
To cite this abstract in AMA style:
Hebing R, Bartelink I, Gosselt H, Lin M, Heil S, De Jonge R, Mathot R. Methotrexate Polyglutamates Exposure – Response Modelling in a Large Cohort of Rheumatoid Arthritis Patients Starting Methotrexate [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/methotrexate-polyglutamates-exposure-response-modelling-in-a-large-cohort-of-rheumatoid-arthritis-patients-starting-methotrexate/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-polyglutamates-exposure-response-modelling-in-a-large-cohort-of-rheumatoid-arthritis-patients-starting-methotrexate/