Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Inflammatory diseases such as rheumatoid arthritis (RA) are associated with oxidative stress as a result of elevated levels of reactive oxygen species (ROS). Oxidative stress leads to the formation of malondialdehyde (MDA) and acetaldehyde (AA), both of which react to form malondialdehyde-acetaldehyde (MAA) protein adducts. Our previous in vitro experiments have demonstrated that methotrexate (MTX) significantly decreases MAA-adduction of proteins (human albumin). Furthermore, the ROS generated by the reaction of MDA+AA to form MAA-adducted proteins are directly scavenged by MTX. However, the biological relevance of MTX’s ability to scavenge ROS and inhibit the generation of MAA-adducted proteins remains unclear. Therefore, we tested the hypothesis that MTX inhibits intracellular redox signaling as well as the formation of MAA-adducted proteins.
Methods: To test our hypothesis, we examined the activation of a well-described redox-sensitive intracellular signaling pathway in which nuclear factor erythroid 2-related factor (NRF2), a redox-sensitive transcription factor, is stabilized and translocates to the nucleus. In the nucleus, NRF2 binds to antioxidant response elements (ARE) by utilizing a NRF2/ARE Luciferase reporter cell line in which luciferase expression is under control NRF2. Cells were incubated with vehicle, Human Serum Albumin (ALB), AA, MDA, MDA+AA (unbound), MAA-Alb for 24 hours at 37oC in the absence or presence of MTX (2μM). In addition, to further evaluate the scavenging of ROS by MTX, tert-butylhydroquinone (TBHQ, 5 mM), a known inducer of NRF2, in the presence of MTX was added to NRF2/ARE cells and incubated for 24 hours. NRF2 activation was quantified by measuring the amount of luciferase via luminescence the cell line produced in response to various stimuli.
Results: As shown in Figure 1. MDA+AA significantly (p<0.001) increased NRF2 activation compared to cells treated with vehicle or Alb controls and MTX significantly attenuated (p<0.001) this redox-sensitive response. NRF2 activation with AA, MDA, or MAA-Alb in isolation demonstrated no increase in NRF activation. Furthermore, as a positive control MTX significantly (p<0.001) inhibited the well-characterized TBHQ-dependent activation of NRF2 by almost 3-fold.
Conclusion: This study demonstrates that when MDA+AA are mixed in the culture, a biologically relevant redox-sensitive signaling pathway is induced, that activates NRF2. Interestingly, the MAA-adduct (MAA-Alb) is unable to activate NRF2, indicating the response is activated through the metabolites of ROS. Furthermore, the ability of MTX to inhibit this redox-dependent cellular response corroborates our previous observation that MTX scavenges ROS. Together, these data strongly indicate a novel mechanism of MTX and suggest a new therapeutic benefit of MTX, which is its antioxidant potential. 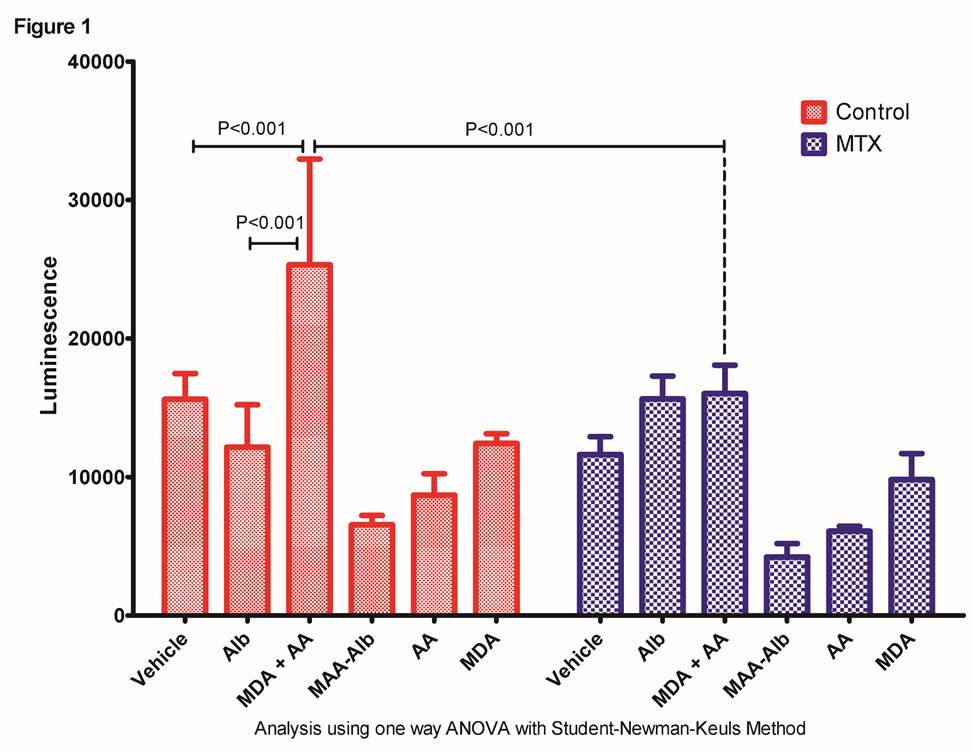
To cite this abstract in AMA style:
Chiou A, Duryee MJ, Sarmiento C, Zimmerman M, Hunter CD, Klassen LW, O'Dell JR, Anderson DR, Thiele GM, Mikuls TR. Methotrexate Inhibits Intracellular Redox Signaling Induced By the Reactive Oxygen Species; Malondialdehyde and Acetaldehyde [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/methotrexate-inhibits-intracellular-redox-signaling-induced-by-the-reactive-oxygen-species-malondialdehyde-and-acetaldehyde/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-inhibits-intracellular-redox-signaling-induced-by-the-reactive-oxygen-species-malondialdehyde-and-acetaldehyde/
