Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Patients with immune mediated inflammatory disorders (IMIDs) have an inherently heightened susceptibility to infection and may be considered high risk for developing COVID-19. While data regarding the COVID-19 vaccine’s immunogenicity in an immunocompetent adult population is rapidly emerging, the ability of IMID patients to adequately respond to these vaccines is not known. Here, we investigate the humoral and cellular immune response to mRNA COVID-19 vaccines in patients with IMIDs on immunomodulatory treatment
Methods: Patients with immune mediated inflammatory disorders (IMIDs) have an inherently heightened susceptibility to infection and may be considered high risk for developing COVID-19. While data regarding the COVID-19 vaccine’s immunogenicity in an immunocompetent adult population is rapidly emerging, the ability of IMID patients to adequately respond to these vaccines is not known. Here, we investigate the humoral and cellular immune response to mRNA COVID-19 vaccines in patients with IMIDs on immunomodulatory treatment.
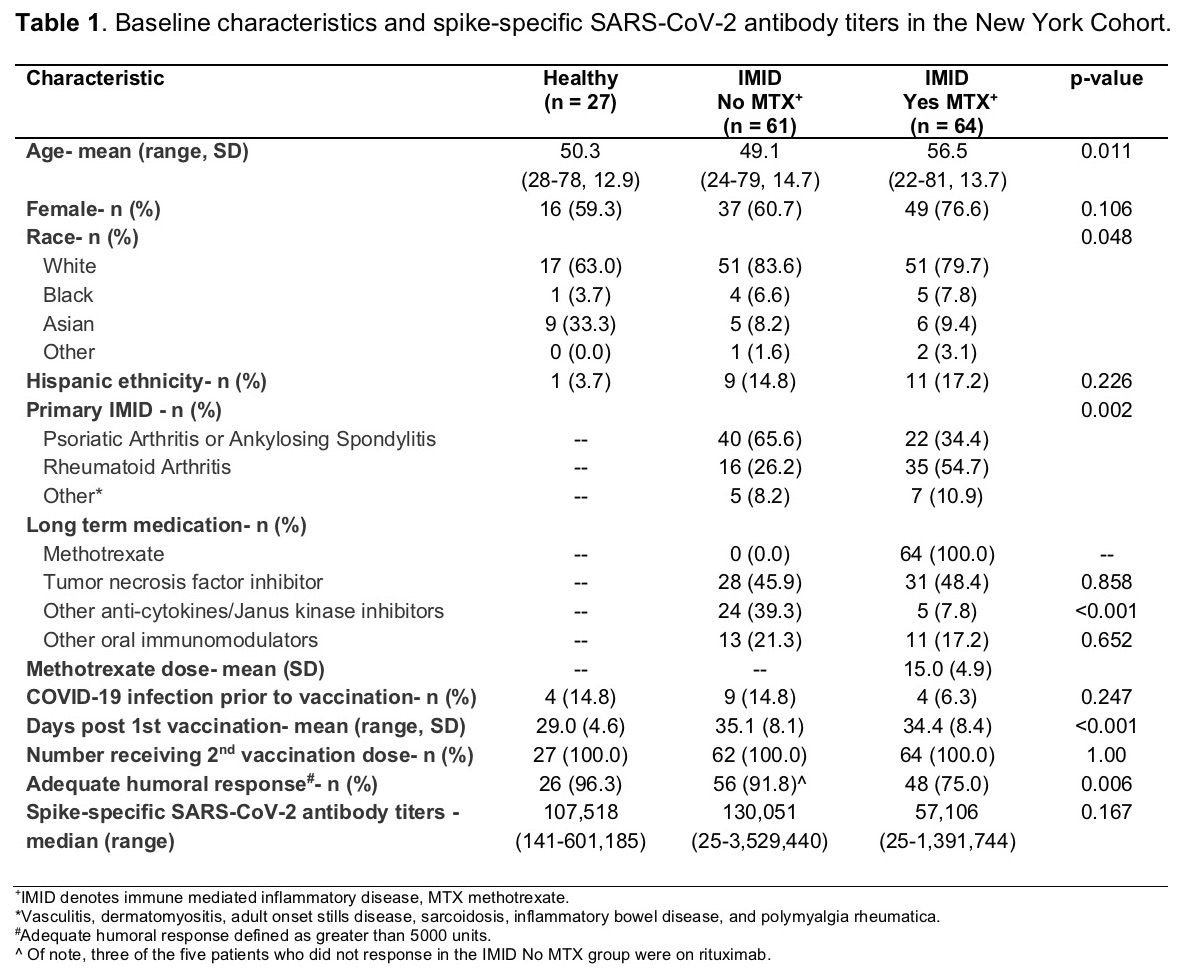
Results: The NY cohort baseline characteristics are found in Table 1. The Erlangen cohort consisted of 182 healthy subjects, 11 subjects with IMID receiving TNFi monotherapy, and 20 subjects with IMID on MTX monotherapy. In both cohorts, healthy individuals and those with IMID not on MTX were similar in age, while those IMID patients receiving MTX were generally older.
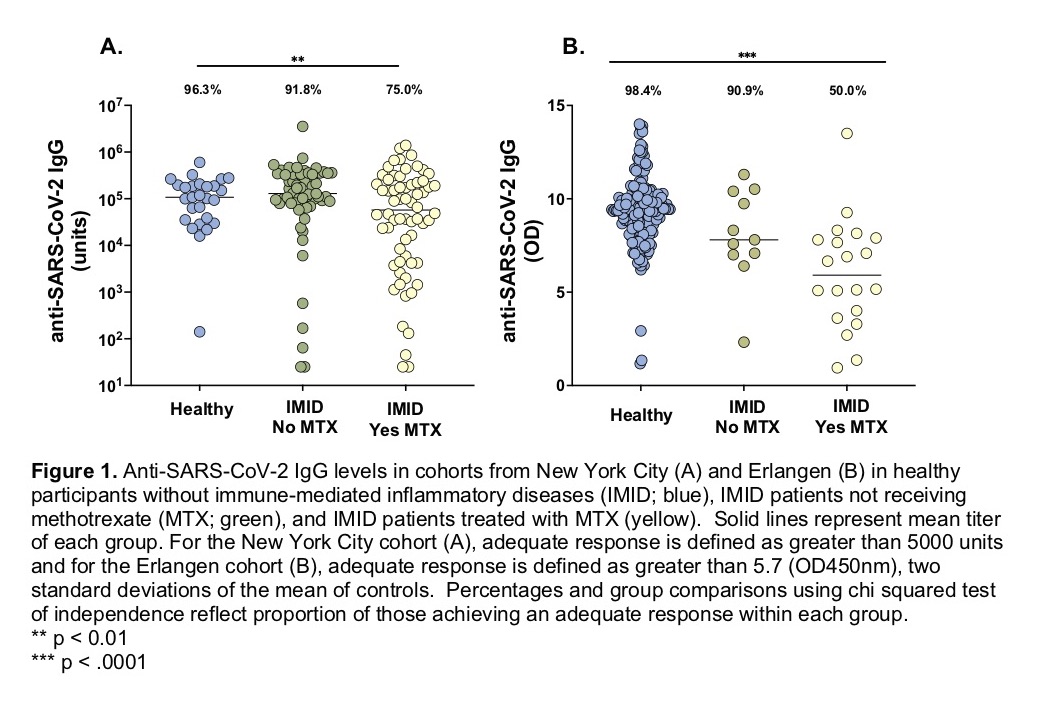
In the NY cohort, of the healthy participants, 96.3% demonstrated adequate humoral immune response. Patients with IMID not on MTX achieved a similar rate of high antibody response rate (91.8%), while those on MTX had a lower rate of adequate humoral response (75.0%) (Figure 1A). This remains true even after the exclusion of patients who had evidence of prior COVID-19 infection (P= 0.014). Of note, 3 out of the 4 IMID patients receiving rituximab did not produce an adequate response. Similarly, in the Erlangen validation cohort, 98.3% of healthy controls, 90.9% of patients with IMID receiving TNFi monotherapy, and 50.0% receiving MTX monotherapy achieved adequate immunogenicity (Figure 1B). These differences remain significant when combining the cohorts, using a stricter definition of adequate response, and in a subgroup analysis by age.
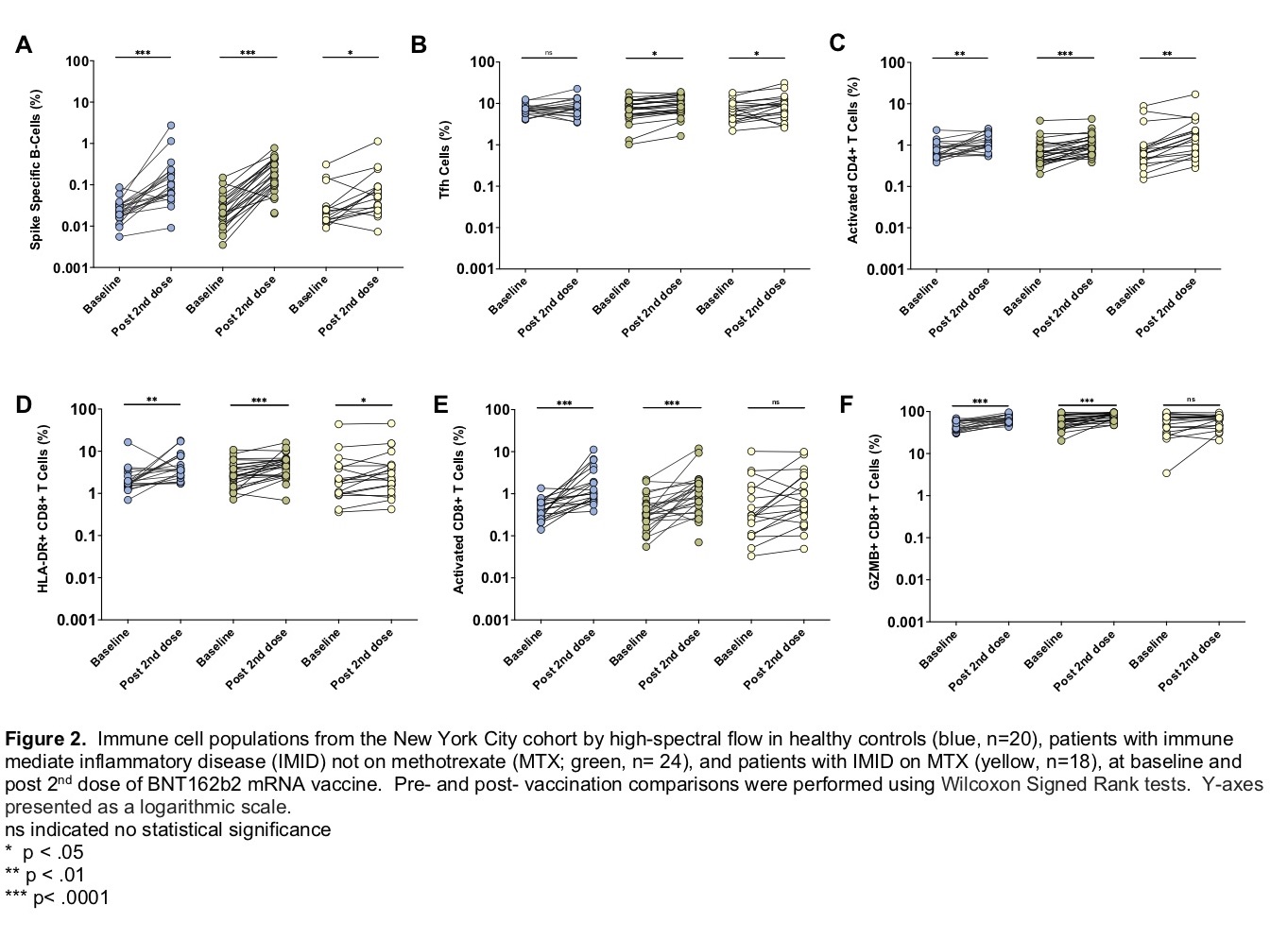
Cellular response was also analyzed in a subgroup of the NY cohort before and after second vaccination. Activated CD8+ T cells (CD8+ T cells expressing Ki67 and CD38) and the granzyme B-producing subset of these activated CD8+ T cells, were induced in immunocompetent adults and those with IMID not on MTX, but not induced in patients receiving MTX (Figure 2).
Conclusion: In two independent cohorts of IMID patients, MTX, a widely used immunomodulator for the treatment of several IMIDs, adversely affected humoral and cellular immune response to COVID-19 mRNA vaccines. Although precise cut offs for immunogenicity that correlate with vaccine efficacy are yet to be established, our findings suggest that different strategies may need to be explored in patients with IMID taking MTX to increase the chances of immunization efficacy against SARS-CoV-2, as has been demonstrated for other viral vaccines.
To cite this abstract in AMA style:
Haberman R, Herati R, Simon D, Samanovic m, Tuen M, Blank R, Koralov S, Atreya R, Tascilar K, Allen J, Castillo R, Cornelius A, Rackoff P, Solomon G, Adhikari S, Azar N, Rosenthal P, Izmirly P, Samuels J, Golden B, Reddy S, Neurath M, Abramson S, Schett G, Mulligan M, Scher J. Methotrexate Hampers Immunogenicity to BNT162b2 mRNA COVID-19 Vaccine in Immune-Mediated Inflammatory Disease [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/methotrexate-hampers-immunogenicity-to-bnt162b2-mrna-covid-19-vaccine-in-immune-mediated-inflammatory-disease/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-hampers-immunogenicity-to-bnt162b2-mrna-covid-19-vaccine-in-immune-mediated-inflammatory-disease/