Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Pediatric
uveitis can lead to ocular complications and vision loss. Treatment consists of
steroid drops, methotrexate (MTX), and anti-tumor necrosis factor (TNF) drugs.
Only 50-75% of children respond to methotrexate. Our aim was to describe the
use and timing of MTX and anti-TNF drugs in pediatric uveitis.
Methods: We reviewed medical
records of 104 children with pediatric uveitis. We collected demographic and
clinical data, and timing of initial MTX use and subsequent anti-TNF use
following uveitis diagnosis. MTX failure was defined as the addition of anti-TNF
agents for the treatment of uveitis. Time to MTX failure was described using
survival analysis modeling strategies.
Results: There were 59 children
with JIA-associated uveitis (JIAU) and 45 with other forms of uveitis (U). Of
these, 85 (82%) were treated with MTX. The majority were female (69%), Caucasian
(61%) or African American (29%). Most had anterior disease (76%), bilateral
involvement (71%), and ocular complications (65%), commonly synechiae (45%),
cataracts (40%), and macular edema (24%). More than half (51% JIAU; 60% U) were
treated with MTX within 6 months of diagnosis. For children with JIAU, MTX was
initiated either prior to uveitis diagnosis (27%) or within 6 months (33%).
Forty
one (48%) required the addition of anti-TNF agents for uveitis at a median of 16
months following MTX use, and 2.2 years following uveitis diagnosis. The
majority (60%) initially received infliximab, and 20% required a second
anti-TNF drug. Kaplan-Meier estimates suggest that 13% need anti-TNF agents
within 6 months of MTX treatment; 16% by 1 year; 36% by 2 years and 59% by 5
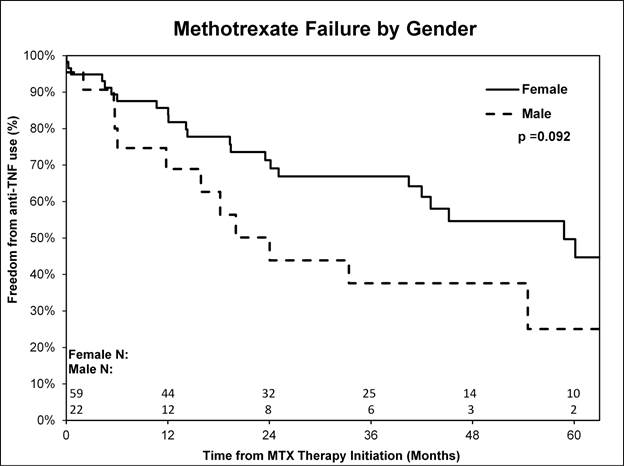
years. Data suggests that one year after MTX therapy, fewer females than males required
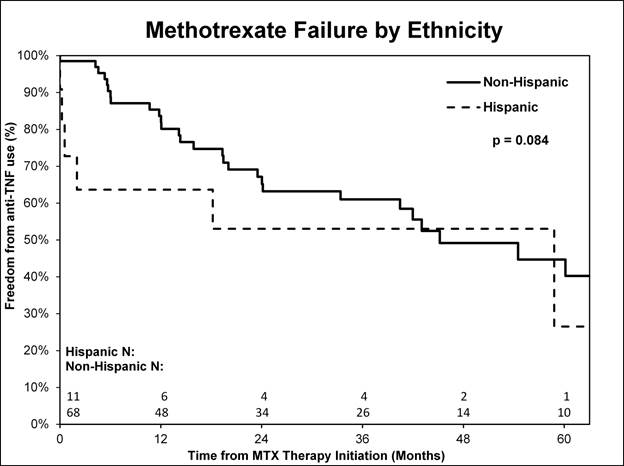
anti-TNF agents (8/59 (14%) vs. 6/22 (27%) p<0.1) [Figure1], and more Hispanic
children were treated with anti-TNF agents compared to non-Hispanics (4/11
(36%) vs. 10/68 (15%) p<0.1) [Figure2]. Timing of initial MTX use following
uveitis diagnosis, type of uveitis, race, ANA positivity, complications, and
age at uveitis diagnosis did not predict timing of MTX failure.
Conclusion: Most children
with uveitis require therapy beyond corticosteroid treatment. Up to 82% are
treated with MTX within 6 months of diagnosis, and almost 50% require an anti-TNF
agent within 2.5 years of diagnosis. One in five children are treated with a second
anti-TNF drug. Male gender and Hispanic ethnicity may be associated with severe
uveitis requiring biologic therapy. Further elucidation of the factors
associated with severe uveitis may help with optimal early treatment of
disease.
To cite this abstract in AMA style:
McCracken C, Yeh S, Jenkins K, Stryker D, Tommasello S, Travers C, Lambert SR, Drews-Botsch C, Angeles-Han ST. Methotrexate Failure in Pediatric Uveitis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/methotrexate-failure-in-pediatric-uveitis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/methotrexate-failure-in-pediatric-uveitis/