Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Juvenile
Idiopathic Arthritis (JIA) is a frequent childhood disease with a prevalence of
1 per 1000 children. The introduction of the biological agents including have
greatly improved the outcome. However a proportion of these children remains
refractory to all of these drugs. We were the first to show earlier that using
another form of cellular therapy, autologous SCT, was effective in some 50% of such
children, even after 8 years of follow up. Given the immunosuppressive effects
of Mesenchymal Stromal Cells (MSC) and clinical responses observed in animal
models and human studies show that MSC could be an attractive and safe option for
this group with the poorest clinical outcome. Infusion of MSC is quite simple
compared to haemopoietic stem cell transplantation. It can be performed as a
short-stay procedure and does not require hazardous treatment with
myelo-ablative drugs. We believe that intravenous injection of MSC in therapy
refractory JIA will be tolerated and will enable us to estimate the effect to
plan for further studies.
Methods: Phase 2 pilot safety study in 6
therapy-refractory JIA patients with a maximum of 3 MSC-infusions according to
the scheme (figure 1). Visit 1 starts at week 0, the next visit (visit 2) will
be after 4 weeks, visit 3 at 8 weeks and so on. The main objective is to offer
a safe alternative for therapy-resistant JIA patients as measured by the total
number of adverse events in the 3 months after MSC infusion compared to 3
months before. Secondary aims: Effectiveness as measured by the active joint
count, by the Juvenile Arthritis Disease Activity Score (JADAS) an by the
erythrocyte sedimentation rate (ESR). Does infusion of MSC induce remission of
inflammation as seen on MRI?
Figure
1.
Results: Currently, 3 of the 6 patients
have been enrolled with now 4 MSC administrations given. Patient 1 is now at
week 16 of the study (visit 4); patient 2 in week 12 (visit 3) and patient 3 in
week 4 (visit 2). No new adverse events were yet observed during or after
administration MSC. In all 3 patients, there was a decline in the number of
active joints at final follow-up compared with study start. The same
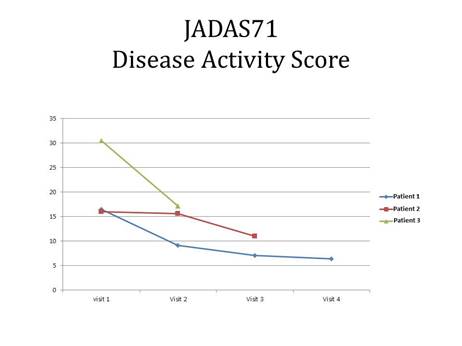
improvement was seen in JADAS (figure 2) with the ESR coming down after each
MSC-infusion. The second follow-up MRI’s of the first two patients had shown
(slight) improvement in inflammation, but the pre-existing cartilage-damage did
not improve.
Conclusion: The preliminary results of a
Phase 2 pilot safety study show that MSC infusion in 3 therapy refractory JIA
patients was safe. Furthermore there was a trend to improvement of the JIA in
active joint count, disease activity score, ESR and improvement as seen on the
MRI of an active joint.
To cite this abstract in AMA style:
Swart J, Wulffraat N, Prakken BJ, Slaper-Cortenbach I, Lindemans C. Mesenchymal Stromal Cell Treatment in Juvenile Idiopathic Arthritis: A Pilot Study [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/mesenchymal-stromal-cell-treatment-in-juvenile-idiopathic-arthritis-a-pilot-study/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mesenchymal-stromal-cell-treatment-in-juvenile-idiopathic-arthritis-a-pilot-study/