Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Eosinophilic granulomatosis with polyangiitis (EGPA) is a severe rare systemic inflammatory disease characterized by elevated blood eosinophil counts ≥1000 cells/µL and vasculitis of small- to medium-sized vessels. Oral corticosteroids (OCS) are a standard treatment; however, high cumulative exposure is associated with adverse events. Mepolizumab, an anti-interleukin-5 monoclonal antibody, has shown efficacy versus placebo in clinical trials, with clinical benefits including remission, reduced relapses and OCS sparing; however, real-world evidence is limited. This study evaluated mepolizumab’s impact on clinical outcomes and medication sparing in EGPA using data from the largest US allergy practice network.
Methods: This retrospective cohort study analyzed structured electronic medical records (EMRs) and unstructured clinical notes from Allergy Partners (01/01/2007–08/17/2023). Patients with EGPA were identified based on diagnosis codes or designation of disease within EMRs, confirmed by physician (allergist)-led chart review, and were aged ≥18 years at mepolizumab initiation (index date) with ≥3 months of clinical activity pre-/post-index. OCS use was evaluated 6 months pre-index and in 6-monthly periods up to 24 months post-index; clinical outcomes: response, control status, remission, sustained stringent remission (no OCS and no relapse) and relapse were assessed ≤24 months pre- versus post-index. Outcomes were stratified by dose (mepolizumab 100/300 mg subcutaneous [overall] or 300 mg at any time). P-values were calculated using Mann‑Kendall Trend tests (OCS use) or McNemar tests (clinical outcomes).
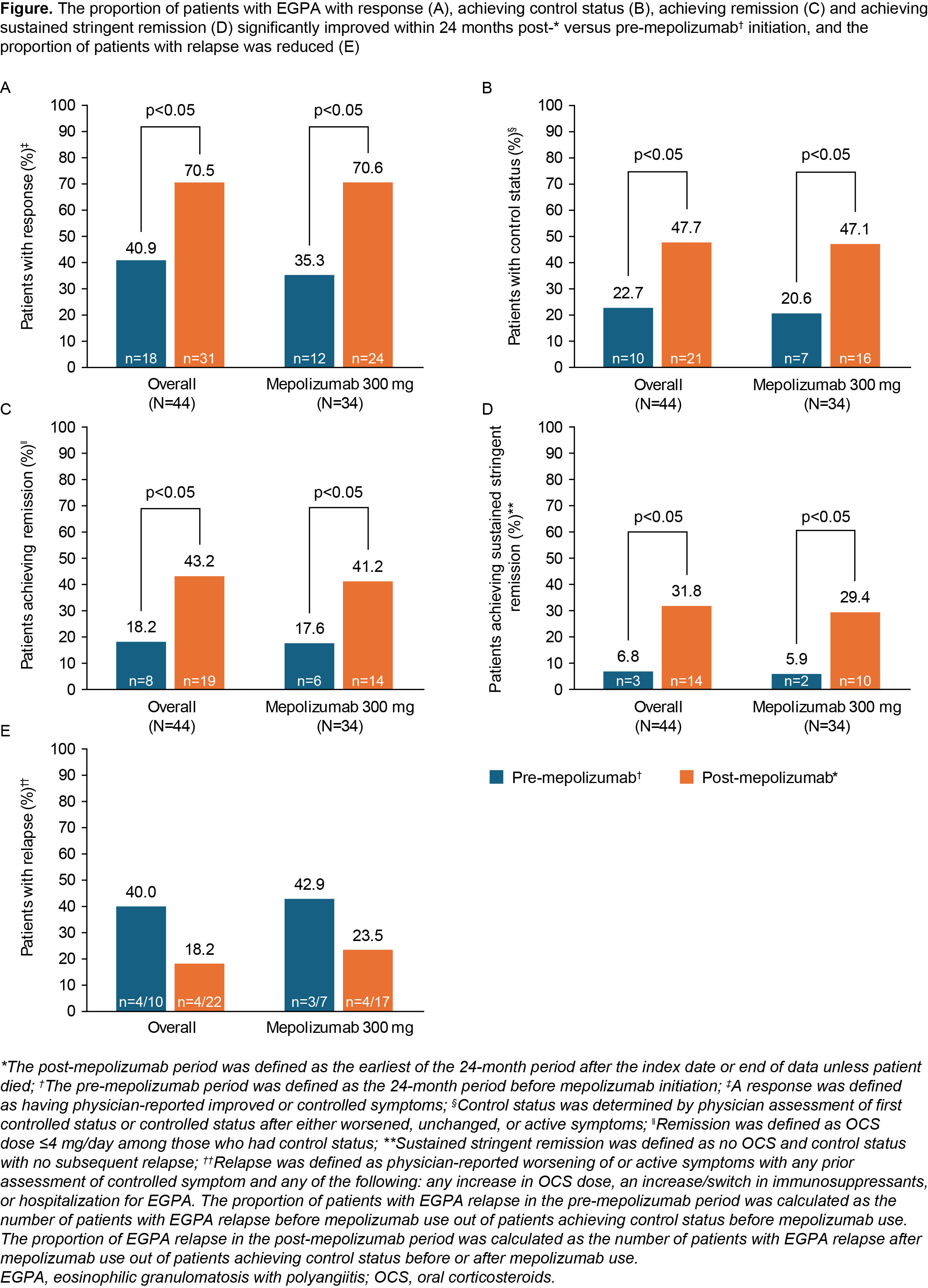
Results: Of the 44 patients included (mean [SD] age: 51.8 [15.2] years; 59.1% female; 40.9% in both vasculitic and eosinophilic disease phases), 30 received mepolizumab 300 mg, 4 switched from 100 to 300 mg and 10 received 100 mg; mean (SD) mepolizumab treatment duration was 2.4 (1.7) years. Overall, 39 patients (88.6%) had a history of OCS use pre-index. There was a significant decrease in the proportion of patients using OCS from 60.5% 6 months pre-index to 25.9% 18–24 months post-index (p=0.014 for trend). Mean (SD) daily OCS dose decreased from 17.5 (23.6) mg/day 6 months pre-index to 5.8 (9.0) mg/day 18–24 months post-index (p=0.014 for trend). Similar significant decreases were observed for these measures in the 300 mg subgroup. In the 300 mg subgroup, there was an increase in the proportion of patients post- versus pre-index with response (70.6 vs 35.3%, p< 0.05), control status (47.1 vs 20.6%, p< 0.05), remission (41.2 vs 17.6%, p< 0.05) and stringent remission (29.4 vs 5.9%); relapses decreased (23.5 vs 42.9%; Figure). Similar benefits were observed for the mepolizumab overall group post- versus pre-index: response (70.5 vs 40.9%, p< 0.05); control status (47.7 vs 22.7%, p< 0.05); remission (43.2 vs 18.2%, p< 0.05); sustained stringent remission (31.8 vs 6.8%, p< 0.05); relapse (18.2 vs 40.0%; Figure).
Conclusion: Mepolizumab offers durable real-world benefits in patients with EGPA with significant OCS sparing, decreased relapse and increased remission post- versus pre-mepolizumab initiation, observed up to 24 months in a large allergy practice setting.
Funding: GSK218960
To cite this abstract in AMA style:
Wechsler M, Kovalszki A, Silver J, Stone B, Seluk L, Huynh L, da Costa Junior W, Ye M, Hwee J, Duh M, McCann W, Edgecomb A. Mepolizumab Treatment Decreased Oral Corticosteroid Use and Improved Clinical Response, Control Status, and Remission in Patients with Eosinophilic Granulomatosis with Polyangiitis: Results up to 24 Months from a Large Network of US Allergy Practices [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/mepolizumab-treatment-decreased-oral-corticosteroid-use-and-improved-clinical-response-control-status-and-remission-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis-results-up-to-24-m/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mepolizumab-treatment-decreased-oral-corticosteroid-use-and-improved-clinical-response-control-status-and-remission-in-patients-with-eosinophilic-granulomatosis-with-polyangiitis-results-up-to-24-m/