Session Information
Date: Sunday, November 7, 2021
Title: Pediatric Rheumatology – Clinical Poster II: SLE, JDM, & Juvenile Scleroderma (0764–0785)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Black and Hispanic children with pediatric lupus (pSLE) have higher morbidity and mortality, but the extent to which differences in outcomes may be related to disparities in treatment, including in medication regimens, is unknown. We aimed to characterize medication use in a large cohort of children with pSLE and determine whether use of steroids, antimalarials and rituximab in pSLE is associated with self-reported race and ethnicity.
Methods: We included patients with pSLE in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry enrolled from 2017-July 2020 with demographic, disease activity and medication data available at baseline visit. Central nervous system (CNS) lupus was defined as documented organic brain syndrome, psychosis, cerebrovascular accident, visual disturbance, cranial neuropathy, or seizure. Large versus small site of care was also included. Summary statistics were used to describe disease and demographic features and medication use in the cohort. We analyzed specific medication use: (1) use versus non-use of antimalarials (2) use of high (≥ 15mg or 0.3mg/kg prednisone equivalent daily) versus low/no prednisone dose at time of baseline visit and (3) use versus non-use of rituximab in the first year of Registry enrollment. Univariate logistic regression analysis was used to identify sociodemographic and disease-related factors associated with each medication outcome. Multivariable logistic regression was used to compare medication outcomes in Black and Hispanic patients to those of non-Black and non-Hispanic patients while adjusting for these covariates.
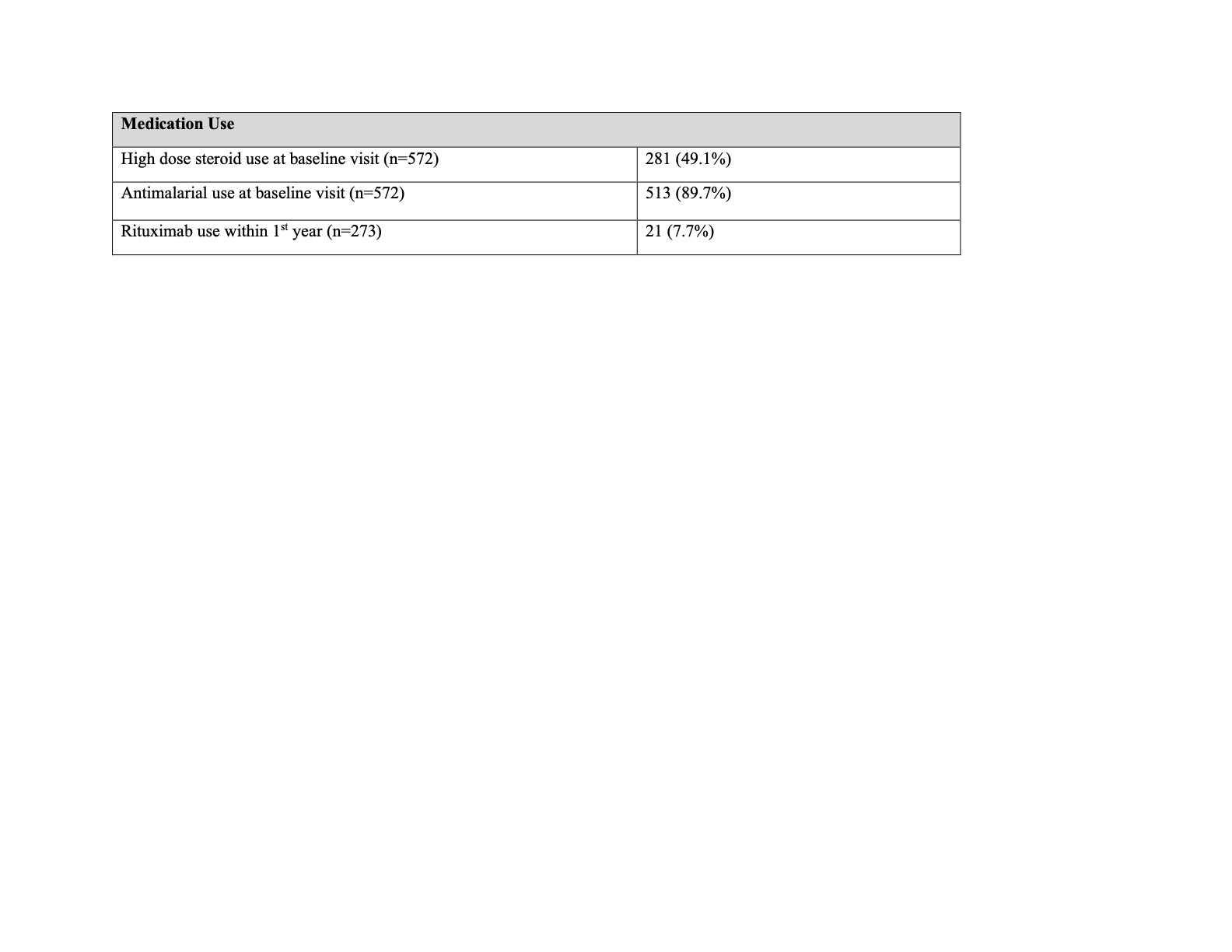
Results: We identified 572 patients with pSLE enrolled in the CARRA Registry with complete data at baseline visit and 273 patients with at least 1 year of follow up. Patient and disease characteristics are presented in Table 1. Median disease duration at baseline visit was 5.8 months. Overall, 90% of patients were prescribed an antimalarial at baseline visit and 49% were on high-dose steroids versus 51% on no or lower-dose steroids (Table 2). Of those with at least 1 year of follow up, 7.7% received rituximab. In multivariate regression, patients with lupus nephritis and those treated at large centers had increased odds of high-dose steroid regimens at their baseline visits; a trend toward higher dose was also seen in lower income patients (Table 3). Black race was associated with over three-fold higher odds of rituximab use within the first year of Registry enrollment even when controlling for disease activity. Antimalarial use at baseline was significantly associated with private insurance.
Conclusion: High dose steroid regimens were associated with higher SLEDAI score, lupus nephritis and large center size. Rituximab use was uncommon and associated with Black race and higher SLEDAI. Antimalarial use was high, though not universal, in this pSLE cohort, and use was associated with private insurance. Further research is needed to understand reasons for this variation and its impact on pediatric lupus outcomes.
To cite this abstract in AMA style:
Roberts J, Berbert L, Son M. Medication Use in Pediatric Lupus in the Childhood Arthritis and Rheumatology Research Alliance Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/medication-use-in-pediatric-lupus-in-the-childhood-arthritis-and-rheumatology-research-alliance-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/medication-use-in-pediatric-lupus-in-the-childhood-arthritis-and-rheumatology-research-alliance-registry/