Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Biologic medications are expensive, and unfortunately their immunogenicity contributes to loss of efficacy over time. Protein particles may form as a result of medication mishandling and are known to enhance the immunologic response to biologics. In order to identify factors contributing to mishandling, we evaluated the temperature excursions and mechanical shock exposures during shipment of biologic medications to patients and described the storage practices of these medications by patients.
Methods: Colorimetric shock indicators registered the highest forces sustained during shipments. During both shipment and patient storage, thermocouple data loggers recorded temperatures every 2 minutes. After medication administration, patients completed and returned a questionnaire along with the data logger. The questionnaire evaluated factors like patient storage habits, refrigerator age, and frequency of adverse medication events. Temperature differences were compared between transit and storage intervals using the Wilcoxon rank-sum test. We also determined if refrigerator location was associated with temperature percent time-in-range and number of excursions out of range under controlled conditions.
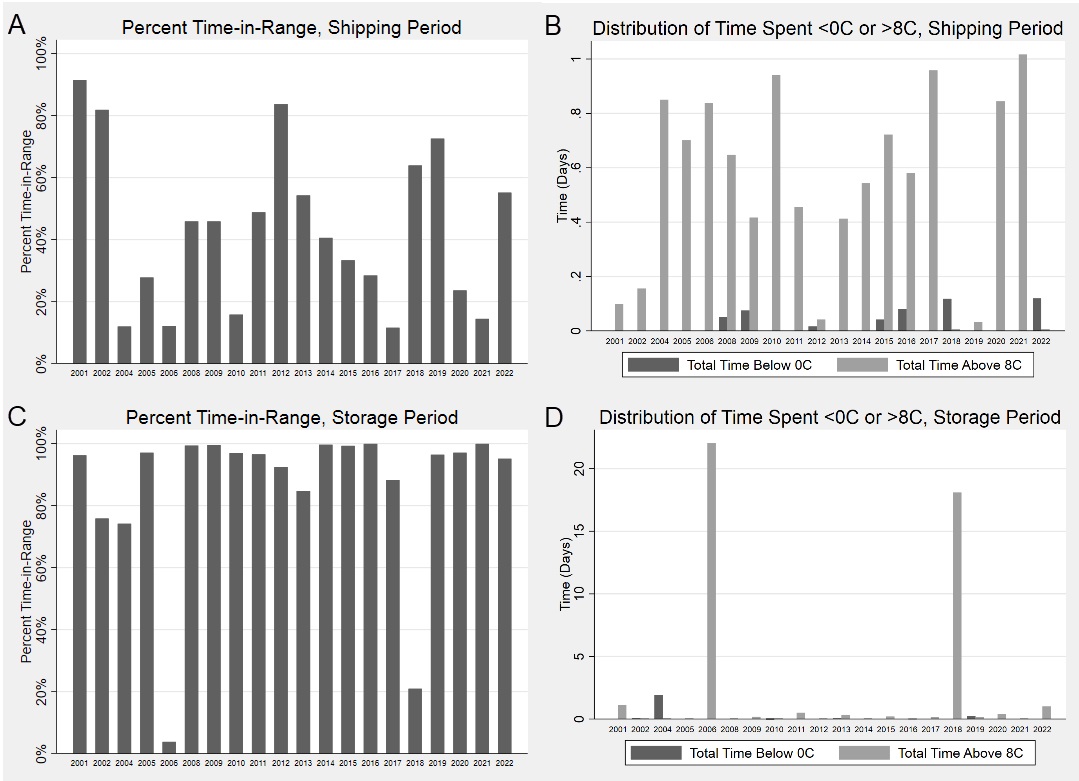
Results: Twenty shipments were analyzed. Shock sensor results showed that 95%, 25%, and 5% of sensors experienced an impact of 25g, 50g and 75g, respectively. Medications were in-range (2-8⁰C) temperatures only 84% of the total time (Fig. 1). The shipping period had an overall percent time-in-range of 43%, compared to 86% during the storage period (Fig 2A,C). In both shipping and storage periods, time spent over 8⁰C was more common than time spent below 0⁰C (Fig. 2B,D). Under controlled experimental conditions for biologic packaging, we identified a relationship between refrigerator location and both percent time in range (p=0.05) and number of excursions (p=0.004) (Fig. 3A,C). The interior rear of the refrigerator near the cold output accounted for this difference with only 68% percent time-in-range and 231 average excursions, (p=0.01 and p=0.004 vs. other locations, Wilcoxon rank sum) (Fig. 3B,D). Dermatologic reactions were the most common adverse event, but there was no association between frequency of adverse events and mishandling (temperature excursions and mechanical shock).
Conclusion: Our current packaging strategy is insufficient both to maintain recommended temperatures during shipping and to mitigate mechanical shocks. Patients maintained biologics at more appropriate storage temperatures compared to temperature control during shipping, but additional patient education may lead to further improvements.
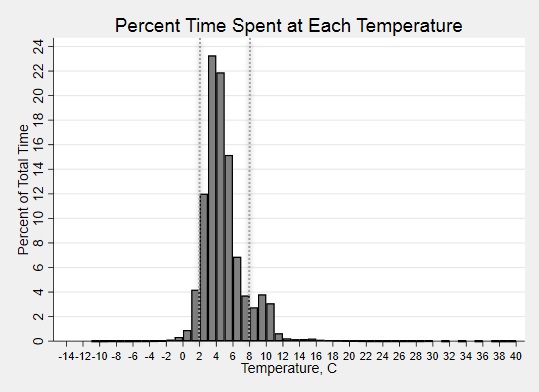 Percent time at each temperature for all trips in aggregate. Dashed lines indicate recommended temperature range.
Percent time at each temperature for all trips in aggregate. Dashed lines indicate recommended temperature range.
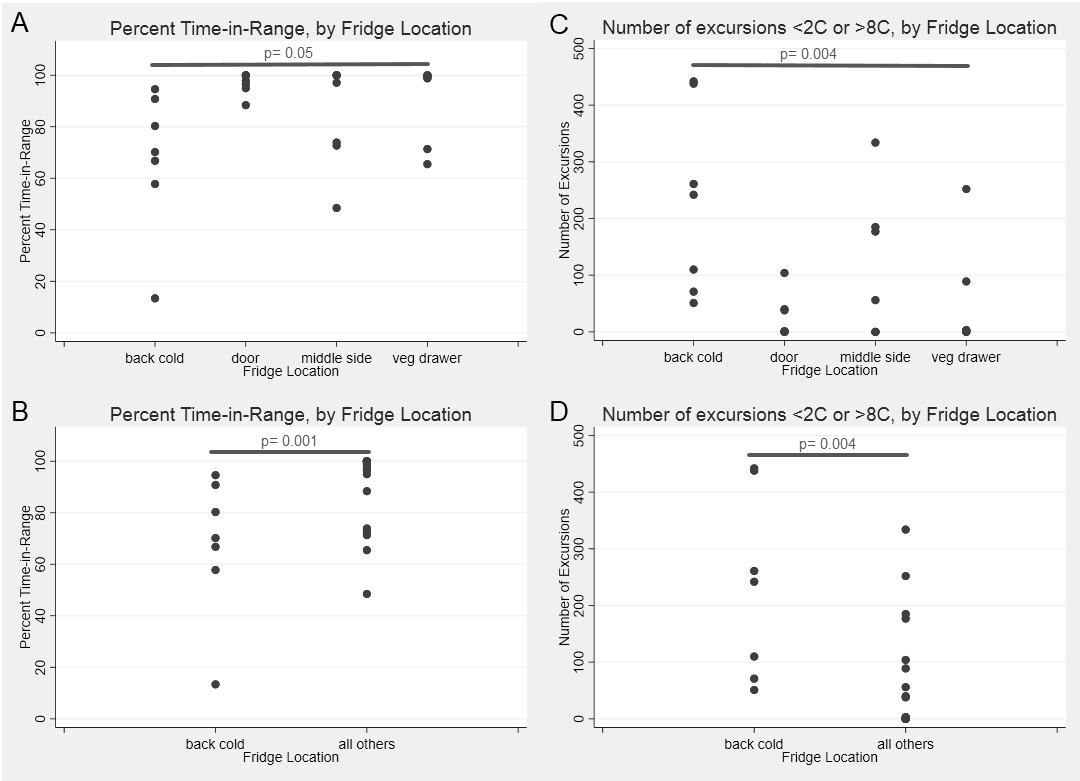 Scatter plots comparing each location overall for percent time-in-range (4A) and number of excursions (4B). Also comparing the back cold (near cold output) to all other areas, percent time-in-range (4C) and number of excursions (4D).
Scatter plots comparing each location overall for percent time-in-range (4A) and number of excursions (4B). Also comparing the back cold (near cold output) to all other areas, percent time-in-range (4C) and number of excursions (4D).
To cite this abstract in AMA style:
Dill S, Cheng E, Brees K, Carpenter J, Caplan L. Mechanical and Temperature Stress During Biologic Shipments to Rheumatology Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/mechanical-and-temperature-stress-during-biologic-shipments-to-rheumatology-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mechanical-and-temperature-stress-during-biologic-shipments-to-rheumatology-patients/