Session Information
Date: Saturday, November 16, 2024
Title: SpA Including PsA – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Un
derstanding the best-performing disease activity measure and response criterion in peripheral spondyloarthritis (pSpA) is crucial for the development of future meaningful clinical studies. The aim of this study was to evaluate the construct validity and discriminatory ability of various instruments to assess components of disease activity and response criteria in patients with pSpA.
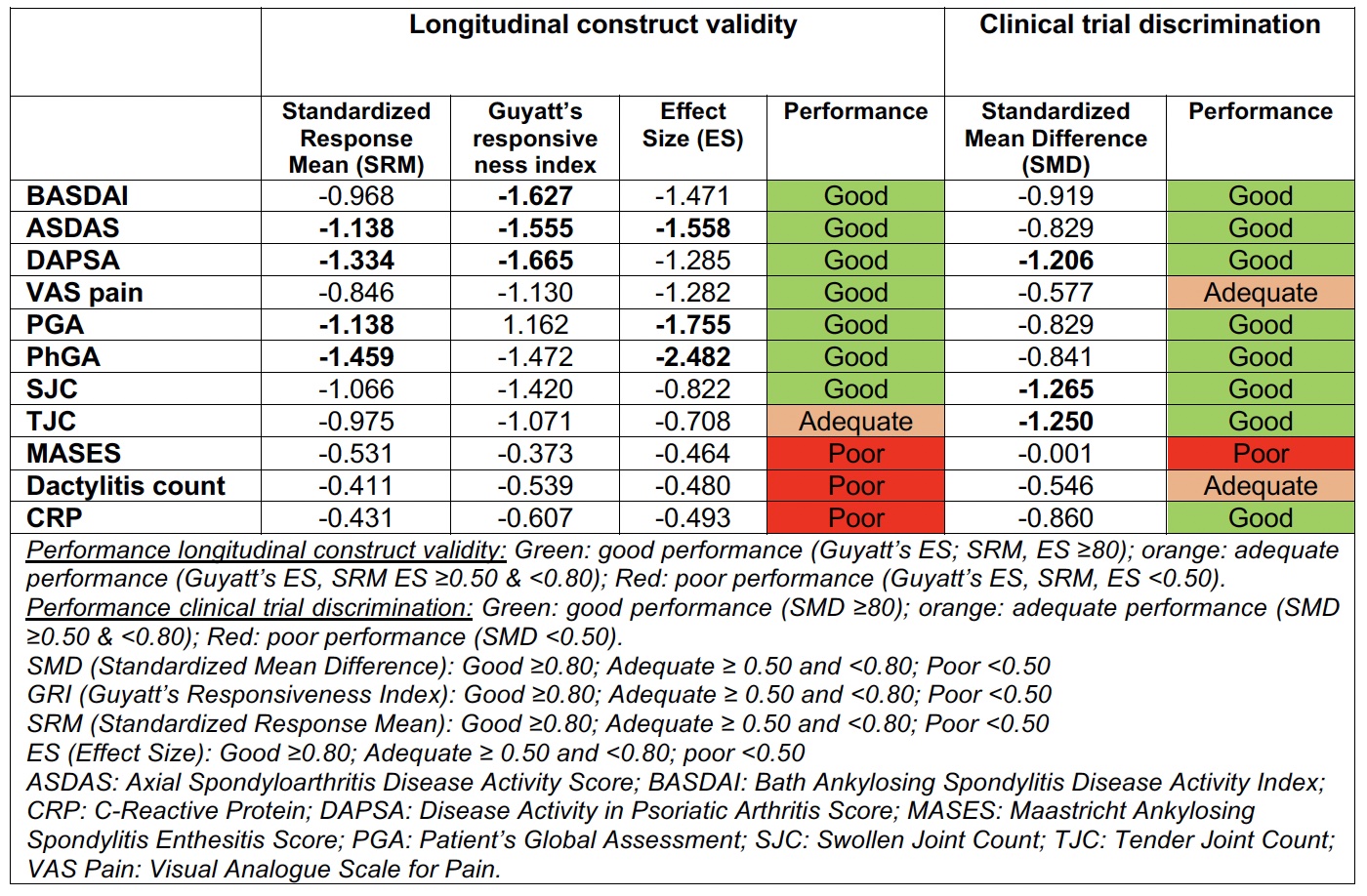
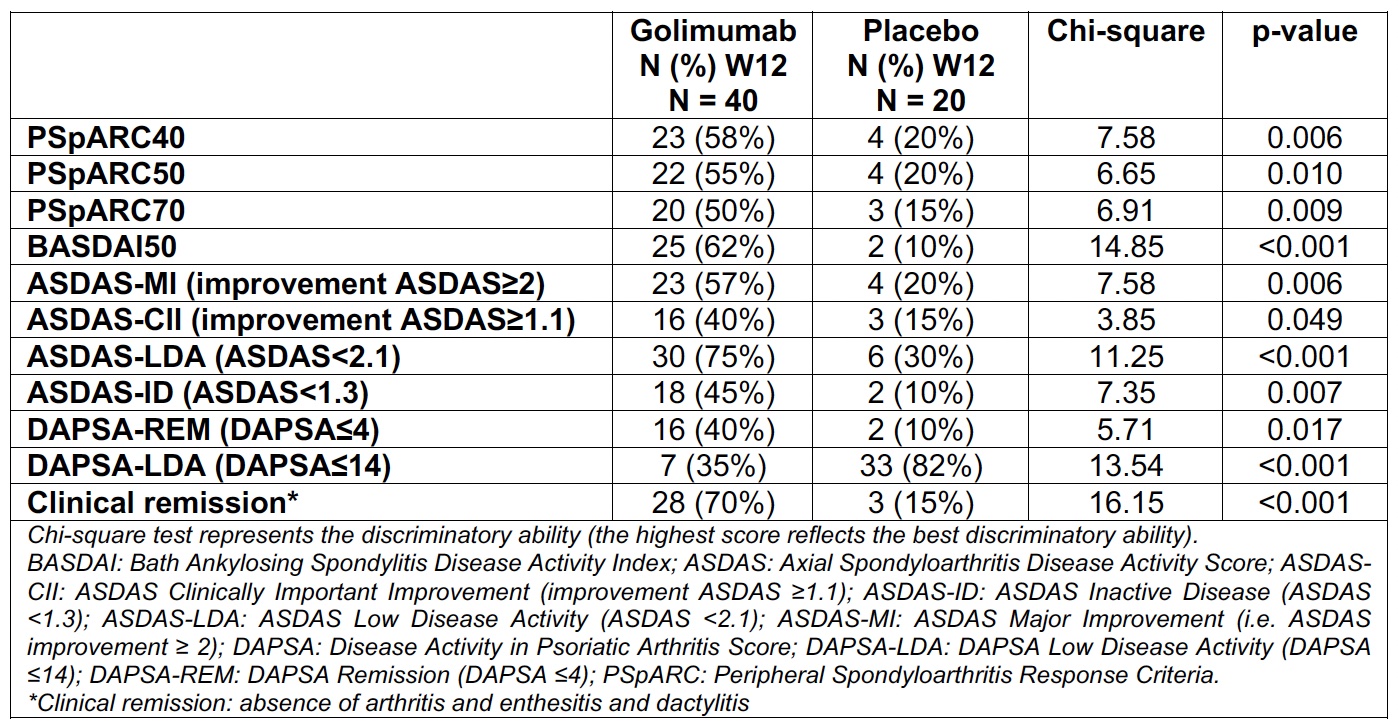
Methods: This is a post-hoc analysis of the CRESPA randomized controlled trial, which included active adult patients newly diagnosed with early pSpA and fulfilling the ASAS classification criteria for pSpA. Patients were randomized to golimumab (GOL) or placebo (PBO) with a 2:1 ratio. The data of the placebo-controlled part of the trial until week 12 were used. Construct validity (known-group discrimination (SMD) in the baseline population), longitudinal construct validity (i.e., Standardized Response Mean (SRM) and (Guyatt’s) effect size) and trial discrimination (standardized mean difference (SMD)) of several continuous disease activity measure instruments (Table 1) were assessed. As part of trial discrimination, Chi-square test was evaluated for binary outcomes.
Results: A total of 60 patients (40 GOL and 20 PBO) were included. Construct validity was better for composite outcomes (i.e. DAPSA, BASDAI and ASDAS), showing a higher SMD between active and inactive patients. Longitudinal construct validity was consistently good for composite outcomes (e.g. ASDAS and DAPSA) and globals (PGA and PhGA), with some differences across methods. On the other hand, clinical trial discrimination was good for both composites (BASDAI, ASDAS, DAPSA) and global (PGA, PhGA) outcomes, as well as for SJC (Table 1).
Finally, among the binary outcomes, trial discrimination was best for clinical remission (defined as absence of arthritis, enthesitis and dactylitis), BASDAI50, DASPA-LDA and ASDAS-LDA (Table 2).
Conclusion: While both composite and global outcome measurement instruments performed well in pSpA, composite scores like DAPSA, ASDAS and BASDAI additionally showed better construct validity. The definition of clinical remission was the most discriminatory response criterion.
To cite this abstract in AMA style:
López Medina C, Molto A, Capelusnik D, Carron P, Webers C, Van den Bosch F, Boonen A, Ramiro S. Measurement Properties of Disease Activity Instruments in Peripheral Spondyloarthritis. an Analysis in the CRESPA Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/measurement-properties-of-disease-activity-instruments-in-peripheral-spondyloarthritis-an-analysis-in-the-crespa-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/measurement-properties-of-disease-activity-instruments-in-peripheral-spondyloarthritis-an-analysis-in-the-crespa-trial/