Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Randomised controlled trials (RCTs) in SLE typically adopt composite responder definitions as primary efficacy endpoints, however outcomes within individual organ domains are also important to understand in order to infer the efficacy profile of a new treatment. The aim of this study was to evaluate how, and how consistently, organ-specific disease activity and therapeutic responses have been measured and reported in lupus RCTs.
Methods: We conducted a scoping review in accordance with the Johanna Briggs Institute Guidance and 2018 PRISMA statement. We systematically searched MEDLINE, EMBASE, Cochrane registry and clinicaltrials.gov. Eligible studies were RCTs investigating efficacy of an immune-directed drug therapy in active SLE, published January 2000-March 2021, excluding studies limited to lupus nephritis. Data items for each eligible RCT were general study/patient characteristics, and clinician-reported outcome measures of organ-specific disease activity at baseline and treatment response definitions at the primary endpoint. Data were extracted independently in duplicate into a pre-established form and summarised descriptively.
Results: A total of 34 RCTs were included (PRISMA diagram, Figure 1). Nine non-renal organ domains were measured using a limited number of instruments. Table 1 summarises the frequency of organ-specific involvement at baseline, and different response definitions used, across the RCTs. Study populations had a high, although variable, frequency of baseline musculoskeletal and mucocutaneous activity and low but also variable representation of other domains. Definitions of organ-specific responses were inconsistent, even within individual instruments. Response in most organ domains were evaluated using BILAG and SLEDAI components but meaningful comparison between treatment arms was limited by small subgroups analysed in post-hoc fashion. Outcome measures using Cutaneous Lupus Erythematosus Disease Area and Severity Index activity scores (CLASI-A) and joint counts (tender, swollen and/or active) were also commonly used, including within pre-specified organ-specific endpoints, which discriminated between treatment arms in some studies.
Conclusion: Mucocutaneous and musculoskeletal manifestations predominate in SLE RCTs. Organ-specific outcome measures are commonly reported, but definitions of involvement and response are inconsistent between trials. Research into the development of new outcome measures for key organ domains, and validation and comparison of response definitions using existing instruments is needed, to facilitate evaluation of the efficacy of novel therapies and ensure the validity, consistency, and comparability of individual organ responses within and between trials.
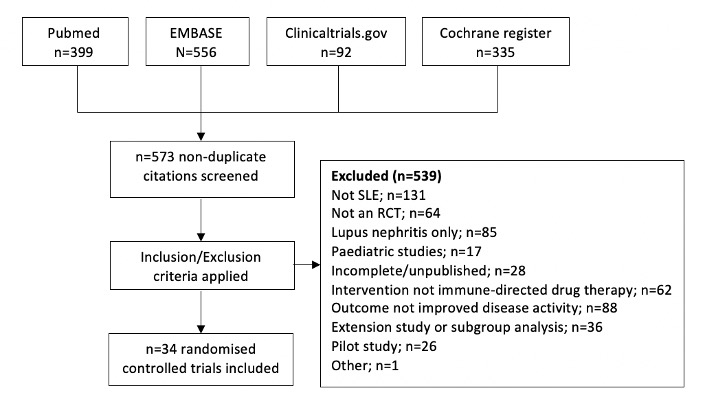 Figure 1: PRISMA diagram demonstrating study selection
Figure 1: PRISMA diagram demonstrating study selection
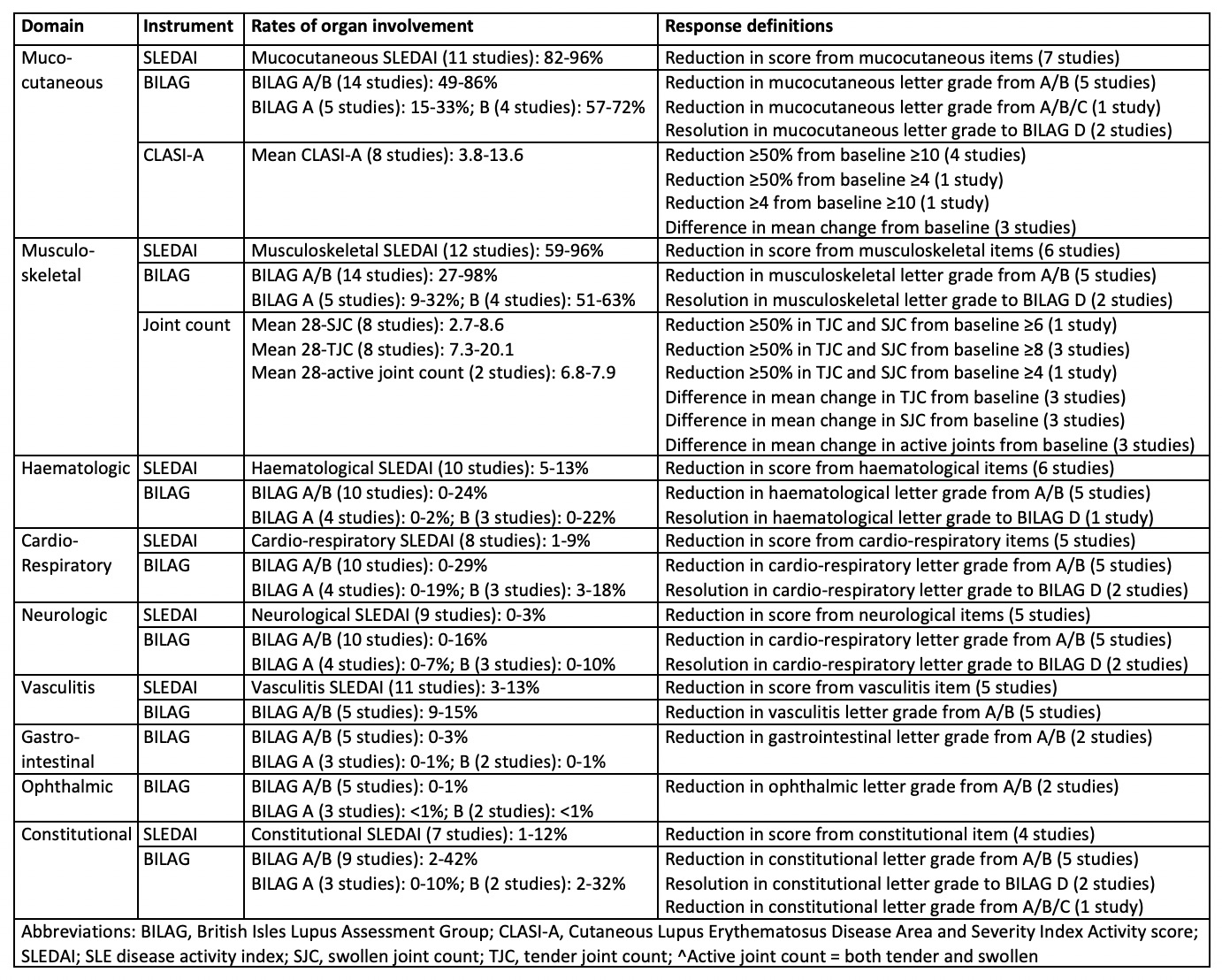 Table 1: Definition and rates of organ-specific activity and treatment responses in the included RCTs, according to instruments employed in at least one study
Table 1: Definition and rates of organ-specific activity and treatment responses in the included RCTs, according to instruments employed in at least one study
To cite this abstract in AMA style:
Connelly K, Vettivel J, Golder V, Kandane-Rathnayake R, Morand E. Measurement of Specific Organ Domains in Lupus Randomised Controlled Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/measurement-of-specific-organ-domains-in-lupus-randomised-controlled-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/measurement-of-specific-organ-domains-in-lupus-randomised-controlled-trials/
