Session Information
Date: Tuesday, November 15, 2016
Title: Spondylarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment - Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The Group for Research of Psoriasis and Psoriatic Arthritis (GRAPPA)-Outcome Measures in Rheumatology (OMERACT) Psoriatic Arthritis (PsA) working group recently obtained endorsement at the OMERACT 2016 conference for the updated PsA core domain set to be measured in PsA clinical trials. Our objective was to integrate patients in each research phase such that the updated PsA core domain set fully reflects a diverse patient perspective.
Methods: The patient voice was sought through an international focus group study covering five continents, two surveys of patients, a consensus meeting with patients and physicians, as well as active partnership of five patient research partners (PRPs) from GRAPPA in the working group. Patient involvement was regularly discussed during reflection meetings by the research team.
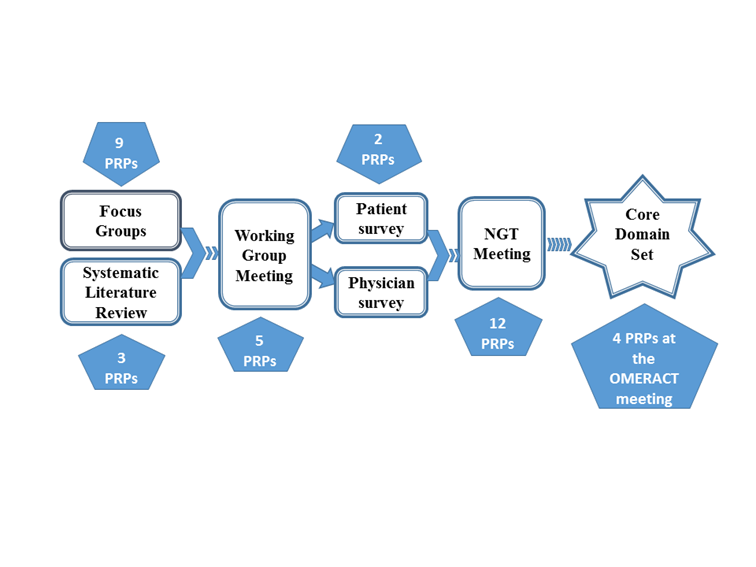
Results: Overall, almost 150 persons with PsA were involved in the study: 1) Systematic literature review. Three PRPs were involved in study protocol development, analysis of results and co-authorship; 2) International focus group study. Ninety patients with PsA participated in 16 focus groups. PRPs gave feedback on the focus group guide, co-moderated focus groups and analyzed and interpreted qualitative data; 3) Surveys. Fifty patients participated in two rounds. Five PRPs participated in the design and analysis of the patient surveys and assisted in writing the introduction text; 4) Consensus meeting. Twelve patients participated in a face-to-face meeting with 12 physicians to agree on a preliminary core domain set. Two PRPs helped in preparing the agenda and patient participant selection.
In total 14 PRPs contributed to one or more of the above research activities. Challenges were identified by physician-researchers (time-constraints and tight deadlines; use of jargon; motivation of PRPs) as well as PRPs (ensuring equal participation and acknowledgement; involvement of pharmaceutical industry).
Conclusion: Collaboration between physician-researchers and PRPs during all core-set development steps enhanced the integration of the patient perspective in a meaningful and representative manner and provided additional face validity. PRP involvement in qualitative data analysis and development of the domain list for the surveys ensured that domains important to patients were maintained throughout the research process and phrased in a manner understandable to patients.
Figure 1. Flow chart of research activities with active participation of patients and patient research partners (PRP) in the update of the OMERACT Psoriatic Arthritis core domain set.
To cite this abstract in AMA style:
de Wit M, Ogdie A, Campbell W, Mease PJ, Goel N, Gossec L, Leung YY, Lindsay C, Palominos P Jr., Steinkoenig I, Grieb S, Orbai AM. Meaningful Involvement of Patients in the Development of a Core Outcome Set for Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/meaningful-involvement-of-patients-in-the-development-of-a-core-outcome-set-for-psoriatic-arthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/meaningful-involvement-of-patients-in-the-development-of-a-core-outcome-set-for-psoriatic-arthritis/