Session Information
Date: Monday, November 13, 2023
Title: (1345–1364) Reproductive Issues in Rheumatic Disorders Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Abatacept is approved for the treatment of moderate to severe rheumatoid arthritis (RA) and psoriatic arthritis. However, there are limited data on the safety of abatacept when used in pregnancy. The purpose of this study was to estimate the incidence/birth prevalence of selected maternal and infant outcomes in pregnancies exposed to abatacept.
Methods: Pregnant women exposed to any dose of abatacept from the first day of the last menstrual period to the end of the first trimester (with or without continued use in pregnancy) and who resided in the U.S. or Canada were enrolled in the Organization of Teratology Information Specialists (OTIS) Abatacept Pregnancy Exposure Registry between 2007 and 2019. Data on maternal characteristics, pregnancy and infant outcomes including major and minor birth defects were collected throughout pregnancy and the first-year post-partum through maternal interviews, medical records abstraction, and dysmorphology examinations.
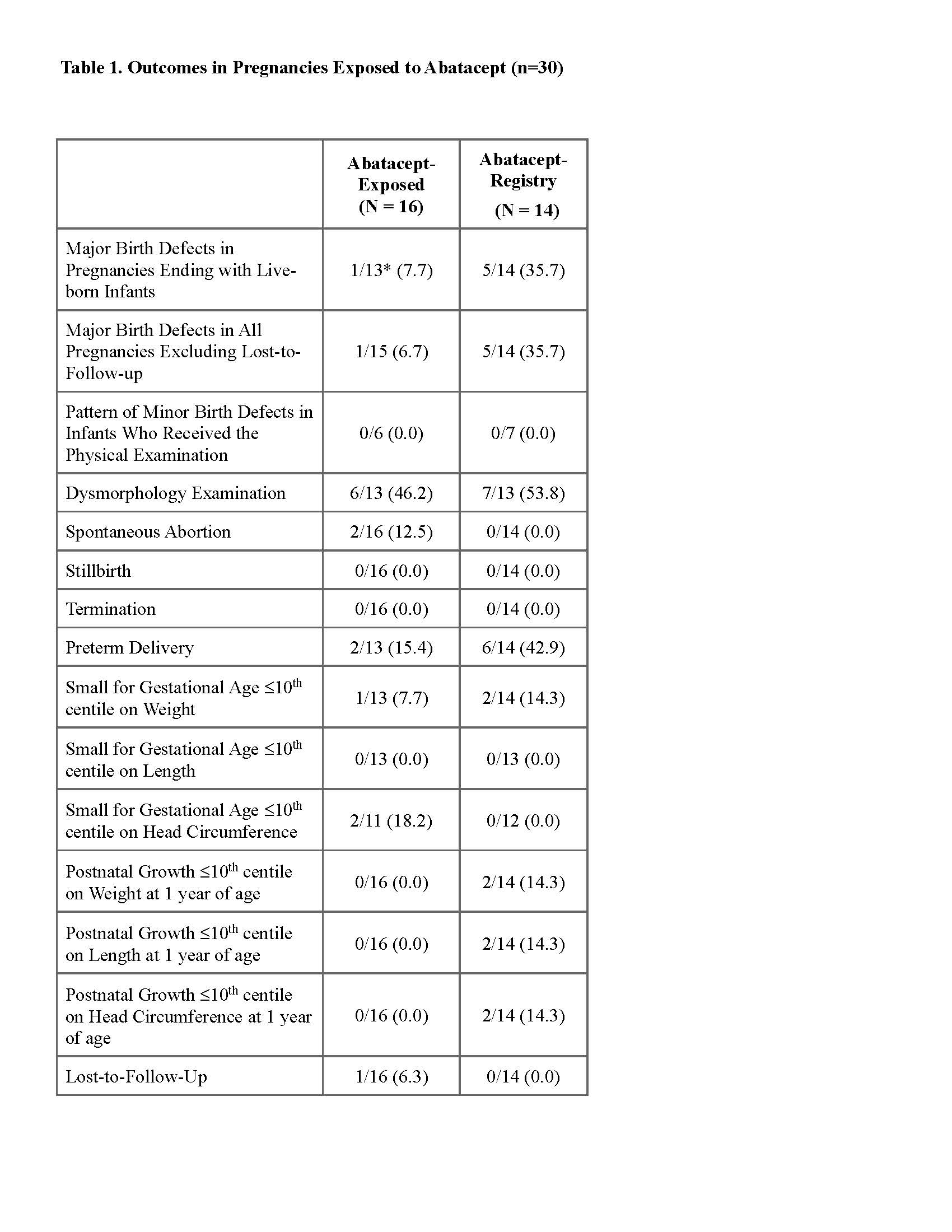
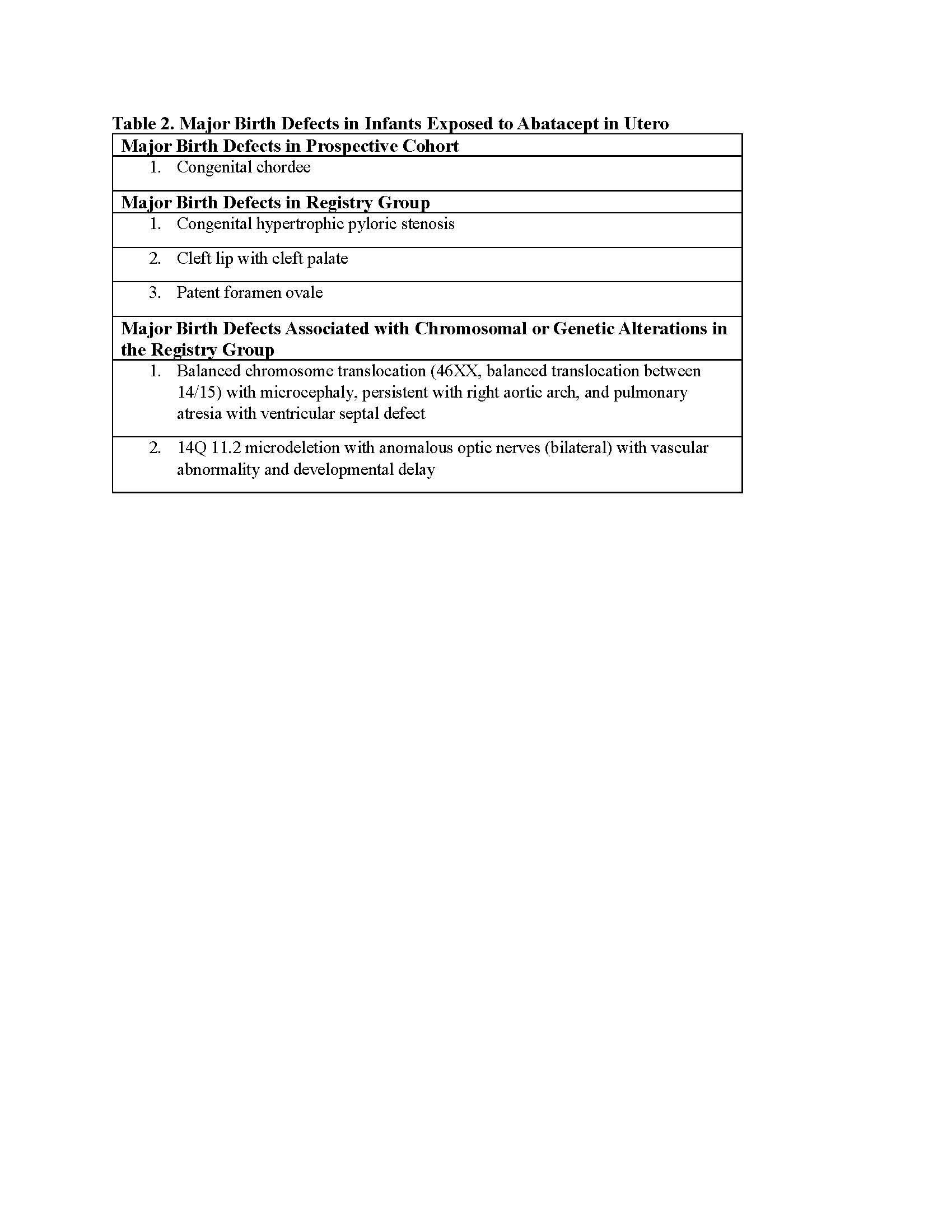
Results: The study sample was comprised of 30 abatacept-exposed pregnancies (Table 1). Of these, 16 were treated for RA and were enrolled in a prospective cohort, and 14 who did not meet the cohort criteria were enrolled in a “registry group”.Reasons that pregnancies did not meet the cohort criteria included retrospective enrollment, co-exposure to an exclusionary medication, or abatacept used for an indication other than RA. In the prospective cohort, 13/16 pregnancies (81.3%) resulted in at least one live birth, 2/16 pregnancies (12.5%) ended in spontaneous abortion, 2/13 pregnancies (15.4%) were delivered preterm ( < 37 weeks’ gestation) and 1/16 (6.3%) was lost-to-follow-up. One pregnancy of 13 ending in a livebirth (8.3%) involved a child with a major birth defect, congenital chordee (Table 2). In the registry group 14/14 pregnancies (100.0%) resulted in at least one livebirth and 6/14 (42.9%) were delivered preterm. Three of 14 pregnancies (21.4%) ended with a liveborn infant with a major birth defect (pyloric stenosis, cleft lip and palate, and patent foramen ovale).An additional two pregnancies in the registry group (14.3%) ended with an infant with major birth defects in association with a genetic or chromosomal alteration (Table 2). A dysmorphology examination for minor birth defects was completed for 6 infants in the prospective cohort and 7 in the registry group. No patterns of either major or minor birth defects were identified. The number of liveborn infants who were small for gestational age (SGA) (≤10th centile for sex and gestational age at birth) or smaller (≤10th centile) at about one year of age on weight, length or head circumference was unremarkable.
Conclusion: Although there was no internal comparison group and the sample size was small, no specific risks were identified in the prospective cohort for any of the outcomes examined. In the registry group, selected adverse outcomes were more frequent, likely due to the biases that led to exclusion of these pregnancies from the prospective cohort. However, no patterns of major or minor birth defects were identified in either group. Future studies with a larger sample size are needed.
To cite this abstract in AMA style:
Caesar M, Johnson D, Jones K, Chambers C. Maternal and Infant Outcomes Following Abatacept Exposure During Pregnancy [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/maternal-and-infant-outcomes-following-abatacept-exposure-during-pregnancy/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maternal-and-infant-outcomes-following-abatacept-exposure-during-pregnancy/