Session Information
Date: Tuesday, October 28, 2025
Title: (2195–2226) Reproductive Issues in Rheumatic Disorders Posters
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Interleukin-17 (IL-17) inhibitors, such as ixekizumab and secukinumab, are increasingly used in the treatment of seronegative inflammatory arthritis, including psoriatic arthritis and axial spondyloarthritis. However, data on their safety and outcomes during pregnancy remain limited. Given the expanding use of IL-17 inhibitors in individuals of reproductive age, further characterization of maternal and fetal outcomes is needed to inform clinical decision-making.
Methods: Nine pregnant individuals with seronegative inflammatory arthritis who were treated with IL-17 inhibitors were identified through the Pregnancy and Rheumatic Diseases Clinics at two Canadian centers. Clinical data were prospectively extracted by a trained research assistant using REDCap, electronic medical records, and relevant consult notes. Outcomes of interest included maternal disease activity during pregnancy, pregnancy complications, neonatal outcomes, and the presence of congenital anomalies.
Results: These patients were managed throughout all trimesters of pregnancy, and followed for six weeks postpartum. The mean maternal age at conception was 34 years. Patient characteristics are summarized in Table 1. No birth defects were observed in any of the infants. One infant was noted to have jaundice (not requiring phototherapy). Another infant required a brief admission to neonatal intensive care unit for hypoglycemia and transaminitis (P7).
Conclusion: In this prospective case series, IL-17 inhibitor exposure during pregnancy was not associated with an increased risk of congenital anomalies or adverse neonatal outcomes. These findings suggest that IL-17 inhibitors may be a safe treatment option for select patients with seronegative inflammatory arthritis during pregnancy. Larger, multicenter studies are needed to confirm these observations and better inform clinical guidance.
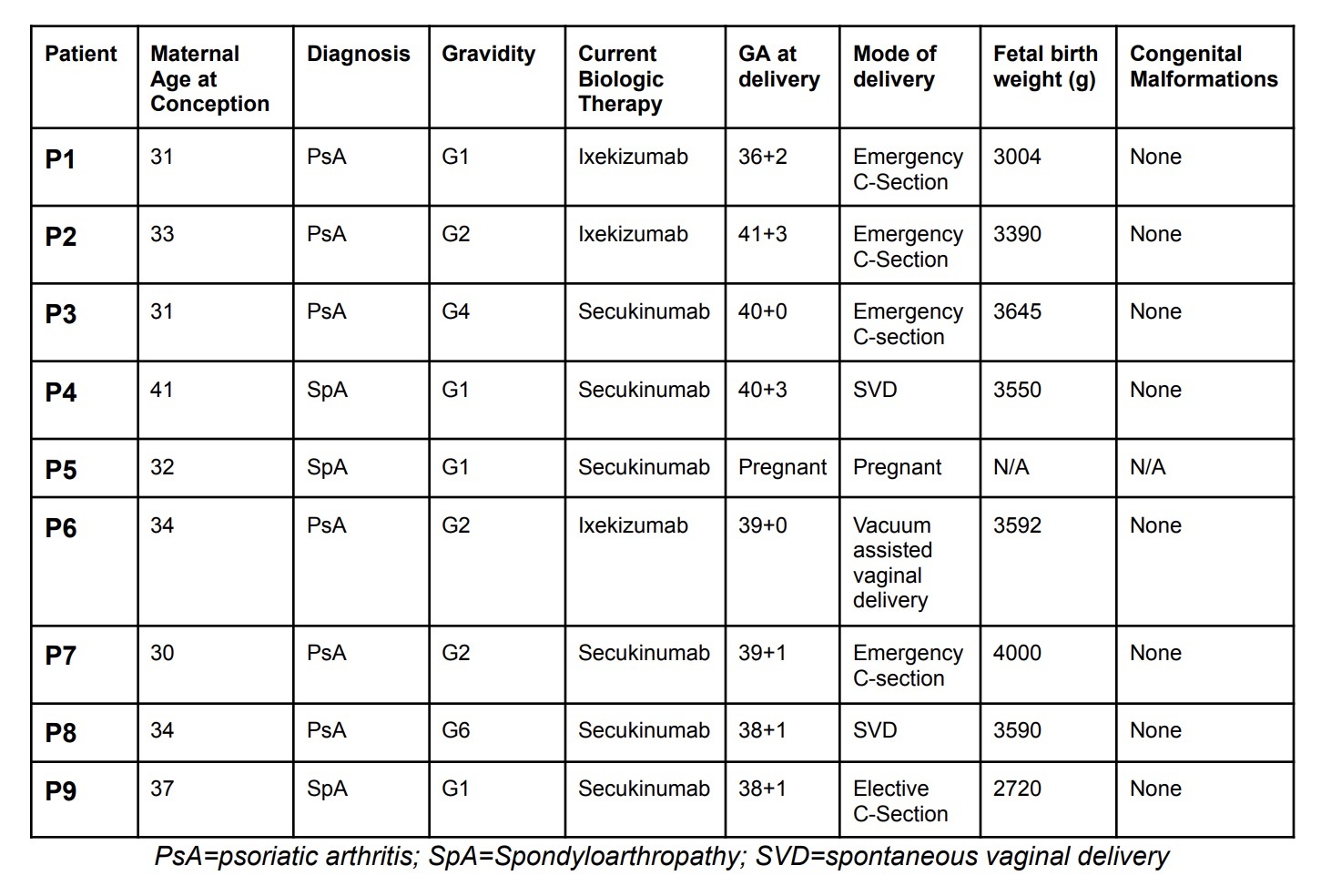 Table 1. Patient characteristics including: maternal age at conception, diagnosis, gravidity, current biologic therapy, gestational age (GA) at delivery, mode of delivery, fetal birth weight, congenital malformations.
Table 1. Patient characteristics including: maternal age at conception, diagnosis, gravidity, current biologic therapy, gestational age (GA) at delivery, mode of delivery, fetal birth weight, congenital malformations.
To cite this abstract in AMA style:
Waring A, Rieger-Torres S, Tan J, Pavlova V, Amiri N. Maternal and Fetal Outcomes Associated with IL-17 Inhibitor Exposure During Pregnancy in Patients with Seronegative Arthritis: A Case Series of Nine [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/maternal-and-fetal-outcomes-associated-with-il-17-inhibitor-exposure-during-pregnancy-in-patients-with-seronegative-arthritis-a-case-series-of-nine/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maternal-and-fetal-outcomes-associated-with-il-17-inhibitor-exposure-during-pregnancy-in-patients-with-seronegative-arthritis-a-case-series-of-nine/
