Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Tumor Necrosis Factor α inhibitors (TNFi) are widely used in patients with RA (Rheumatoid Arthritis) undergoing orthopedic surgery, yet its optimal perioperative management is unknown. The objective of this study is to systematically review the available literature regarding perioperative TNFi management and post-operative infections and to formulate clinical practice recommendations for the optimum perioperative use of TNFi.
Methods:
A librarian assisted search was conducted in PUBMED, EMBASE, and the Cochrane Central Register of Controlled Trials, with defaulted date range of each database using the following key terms: Rheumatoid Arthritis, TNF- α , antirheumatic agent, surgical site infections (SSI), surgery/infection, arthroplasty, anti-TNF- α , infliximab, etanercept, adalimumab, risk factor, perioperative, postoperative. Studies were included if most patients had RA, age ≥ 18, and were undergoing orthopedic surgery. The intervention was use of TNFi. The comparison group was patients not treated with TNFi. The outcome of interest was surgical site infection. Study quality was assessed using Oxford Center for Evidence Based Medicine Levels of Evidence. No randomized controlled trials were available; high quality cohort studies (2b) and case control studies (3b) were included.
Results:
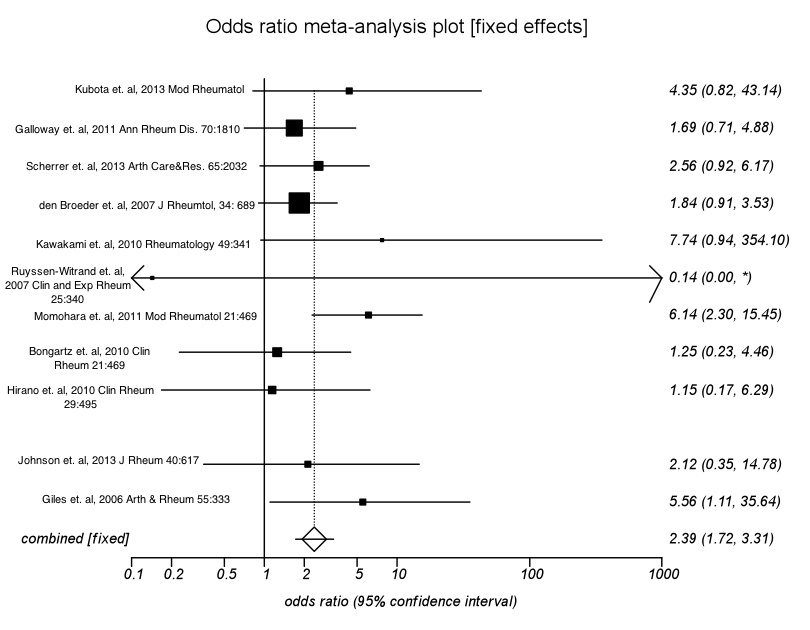
A total of 2,004 studies were found. After abstract review, 30 studies met inclusion criteria. After detailed quality assessment, 11 studies met criteria with low risk of bias, representing 3730 RA patients with recent exposure to TNFi’s (TNF+) and 4,307 with no recent exposure to TNFi’s at the time of surgery (TNFi-). These studies were included in the final analysis. There was no consistent reporting of corticosteroid use. If the non-combinability p-value was greater than 0.20, a fixed-effects model was used to estimate the pooled odds ratio (OR); if p≤0.20, a random effects model was used to estimate the pooled odds ratio (a forest plot is presented).
Patients in the TNF+ group for all orthopedic surgeries had a 2.39-times greater likelihood of developing SSI compared to patients in the TNF- group (pooled fixed-effects OR=2.39; 95% CI=1.72, 3.31; p<0.0001). A smaller group of cases with only total hip and total knee replacement were also meta-analyzed; here patients in the TNF+ group had a 3.08-times greater likelihood of developing SSI compared to patients in the TNF- group (pooled random-effects OR=3.08; 95% CI=0.87, 10.95; p=0.08). The Begg-Mazumdar test and Egger test did not reveal any evidence of publication bias (p = 0.88 and p=0.91, respectively).
Conclusion: Perioperative exposure to TNFi is associated with a higher risk of infection in all orthopedic surgery, although the risk in total hip and knee replacement is less clear. These data support withholding TNFi prior to orthopedic surgery.
Disclosure:
S. M. Goodman,
None;
I. Menon,
None;
R. Smethurst,
None;
P. Christos,
None;
V. P. Bykerk,
Amgen,
5,
Bristol-Myers Squibb,
5,
Pfizer Inc,
5,
UCB ,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/management-of-perioperative-tumor-necrosis-factor-%ce%b1-inhibitors-in-rheumatoid-arthritis-undergoing-arthroplasty-a-systematic-review-and-meta-analysis/