Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Filgotinib (FIL) is a preferential oral Janus kinase (JAK) 1 inhibitor, approved for the treatment of RA and ulcerative colitis (UC) in Europe, the UK, and Japan.1,2 Data from the ORAL Surveillance post-marketing study (NCT02092467) suggest that in patients with active RA aged ≥50 years with ≥1 cardiovascular risk factor, malignancy risk is higher with the pan-JAK inhibitor tofacitinib vs TNF inhibitors.3
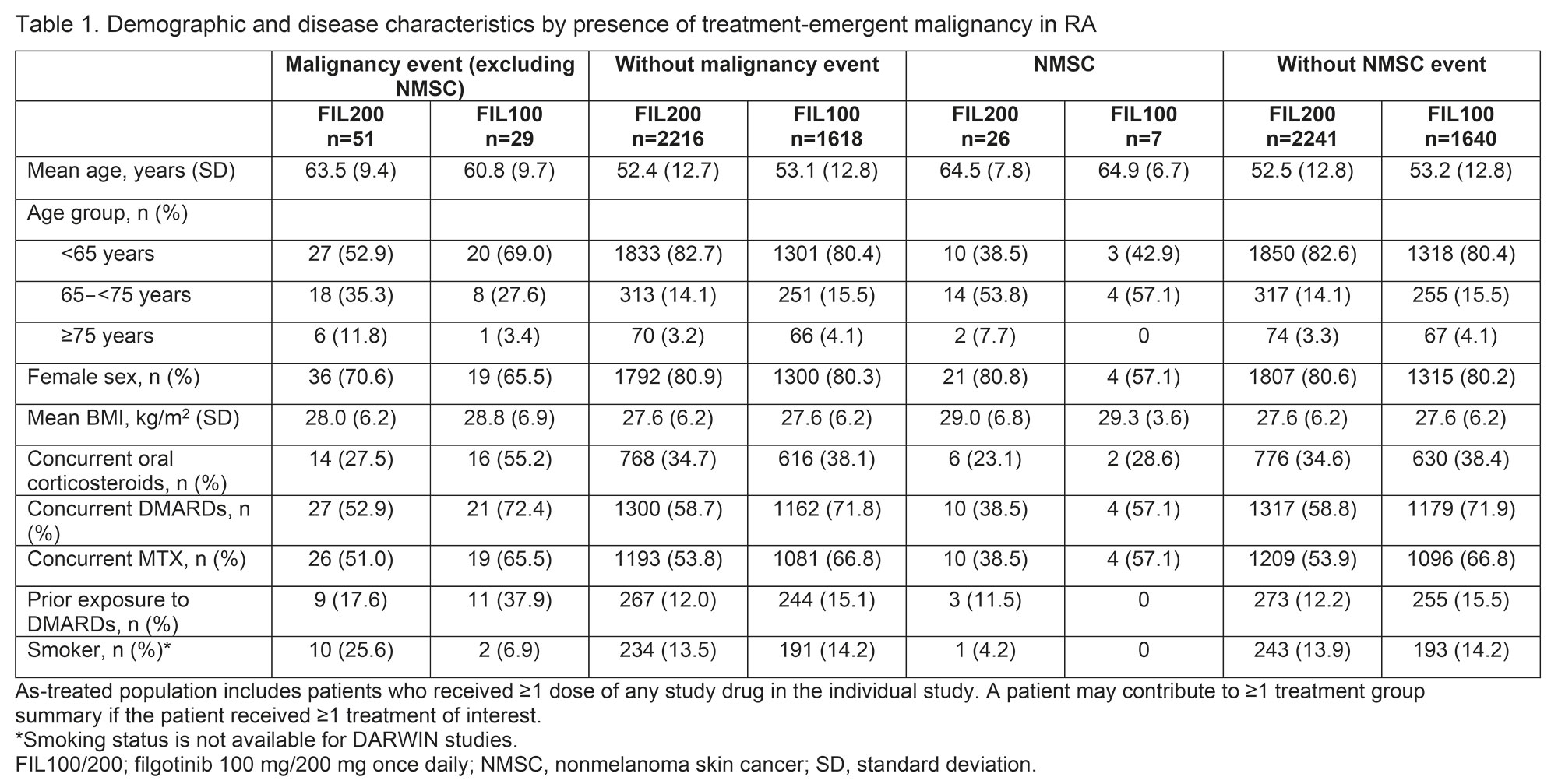
Methods: We assessed malignancy incidence and event rates in clinical trials of FIL 100 mg and 200 mg (FIL100/FIL200) for RA (DARWIN 1–3 [NCT01888874, NCT01894516, NCT02065700] and FINCH 1–4 [NCT02889796, NCT02873936, NCT02886728, NCT03025308]) and UC (MANTA [NCT03201445], SELECTION [NCT02914522], SELECTION LTE [NCT02914535]). Data were as of Jan 11, 2022 (DARWIN 3) and Jan 31, 2022 (FINCH 4) (median FIL exposure: 3.6 years). Analyses were performed on an ad hoc interim analysis data set without additional cleaning. For patients with RA, baseline characteristics were compared in those with vs without malignancies (excluding nonmelanoma skin cancer [NMSC]) and in those with vs without NMSC (the baseline characteristics comparison was not analyzed for UC). Malignancy incidence and event rates in RA and UC were assessed according to treatment group and were compared between patients aged < 65 and ≥65 years.
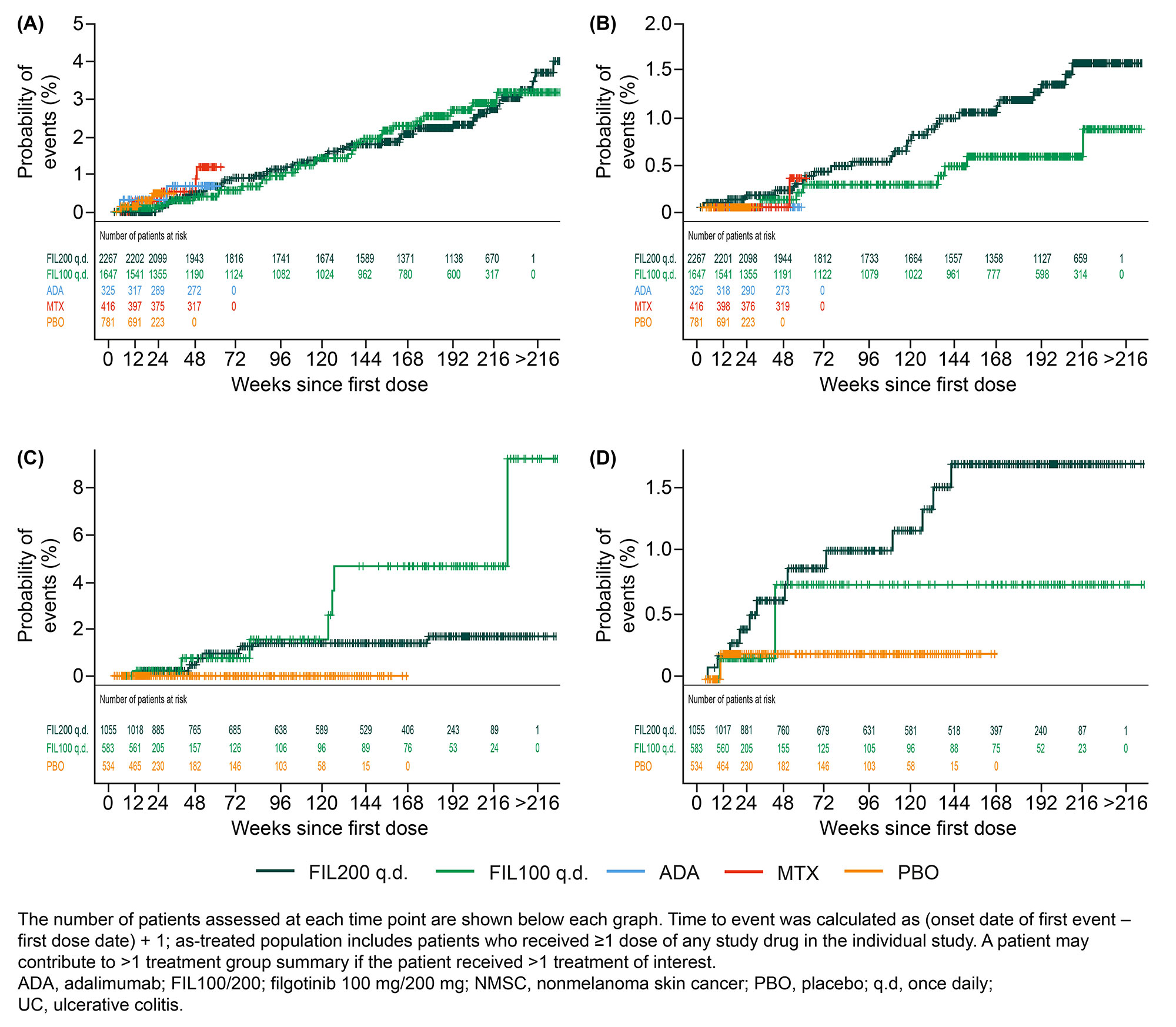
Results: There was a total of 100 malignancy events and 40 NMSC events in patients with RA. The mean patient age was higher in those with vs without malignancies and in those with vs without NMSC (Table 1). Smoking status and concomitant MTX use were not predictors of malignancy or NMSC events. In patients with RA, the exposure-adjusted incidence rate (EAIR) of malignancies and NMSC (Table 2) did not differ between the 2 FIL doses. The exposure-adjusted event rates (EAERs) were similar to EAIRs. There was a total of 20 malignancy events and 20 NMSC events in patients with UC. In patients with UC, the EAIR of malignancies was numerically higher with FIL100 vs FIL200 and the EAIR of NMSC was numerically higher with FIL200 vs FIL100. EAERs were similar to EAIRs. The Figure shows the probability of events.
Patients aged ≥65 years were more susceptible to developing malignancies and NMSC. In this age group, the EAIR (95% confidence interval [CI]) of malignancies was numerically higher with FIL200 vs FIL100 in RA (2.0 [1.3, 3.0] vs 1.0 [0.5, 2.0]) but not in UC (2.3 [0.6, 5.8] vs 3.3 [0.1, 18.5]). Patients aged ≥65 years had higher EAIR (95% CI) of NMSC with FIL200 than with FIL100 in RA (1.4 [0.8, 2.2] vs 0.5 [0.1, 1.2]) but not in UC (3.0 [1.0, 6.9] vs 3.9 [0.1, 21.7]). In both RA and UC, gastrointestinal neoplasms and basal cell carcinoma were the most frequent type of malignancy and NMSC, respectively.
Conclusion: As expected, the risk of malignancies and of NMSC was higher in those aged ≥65 vs < 65 years. There was a numerically higher incidence of malignancies and of NMSC in patients aged ≥65 years with RA treated with FIL200 than with FIL100 but not in those with UC. Longer-term pharmacovigilance is warranted to establish the safety of JAK inhibitors in those with chronic inflammatory diseases.
References
1. Jyseleca SmPC. Galapagos NV; May 2022
2. Jyseleca Japanese PI. Gilead Sciences K.K.; Sep 2020
3. Ytterberg SR, et al. N Engl J Med 2022;386:316-26
To cite this abstract in AMA style:
Mariette X, Aspeslagh S, Moriggl R, Rajendran V, Rudolph C, Van Hoek P, Verbruggen N, Watson C, Borchmann S, Stallmach A. Malignancy Events in the Filgotinib Rheumatoid Arthritis and Ulcerative Colitis Clinical Development Programs [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/malignancy-events-in-the-filgotinib-rheumatoid-arthritis-and-ulcerative-colitis-clinical-development-programs/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/malignancy-events-in-the-filgotinib-rheumatoid-arthritis-and-ulcerative-colitis-clinical-development-programs/