Session Information
Date: Wednesday, October 24, 2018
Title: 6W018 ACR Abstract: Epidemiology & Pub Health IV: Determinants & Consequences of Tx (2952–2957)
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: NSAIDs and Coxibs represent one of the most commonly prescribed drugs by rheumatologists and are used regularly by >10 million Americans. While most patients enjoy analgesic benefits with these medications, some experience major toxicities. Improving the risk-benefit ratio requires a more precise understanding of the risks for an individual patient. The goal of this research was to derive and validate a risk score for major NSAID toxicity.
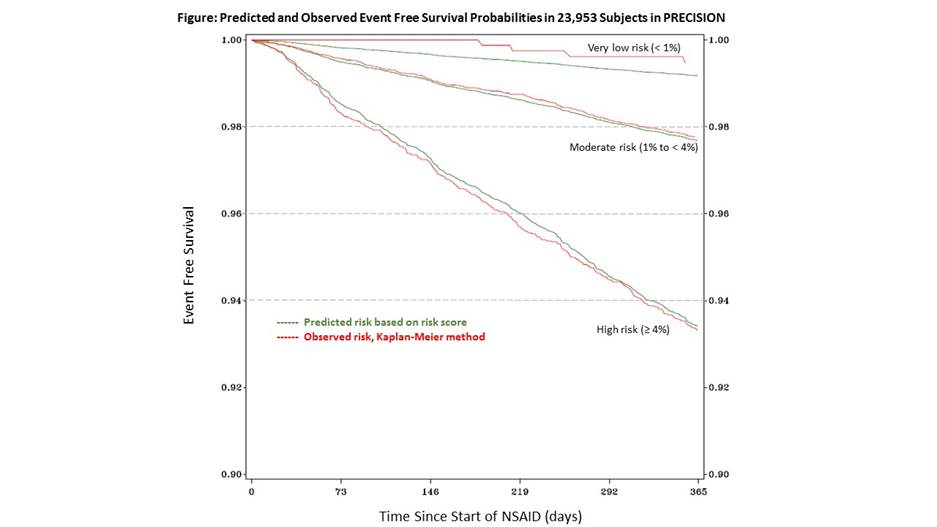
Methods: The TRIPOD recommendations were followed for conducting this research, such that patients recruited during the first 4 years (n = 15,196) of enrollment in the PRECISION trial were used to derive a risk score and patients enrolled in last 5 years (n= 8,757) were used for validation. Participants were censored at 1 year, patient termination of study NSAID or time of first major toxicity. The major NSAID toxicity outcomes included cardiovascular (CV) event, clinically significant gastrointestinal event, significant renal events, or death. Variables significantly associated (p<0.1) with major toxicity after adjustment for baseline age and gender were candidates for inclusion in the final Cox proportional hazards regression model. Discrimination of the model was assessed with the c-index and calibration was assessed by examining the slope from the observed to expected risk plots. After derived models were found to have similar model fit statistics in the validation set, the cohorts were combined, allowing calculation of a risk score for the 1-year probability of major NSAID toxicity. Three risk categories were created: very low (<1%), moderate (1 to <4%) and high (4+%); agreement was assessed between predicted and observed risk.
Results: In the derivation cohort, statistically significant variables included age (HR 1.03 per year), male sex (HR 1.51), history of CVD (HR 1.94), history of hypertension (HR1.29), history of diabetes (HR 1.42), aspirin use (HR 1.37), tobacco use (HR 1.55), statin use (HR 1.43), elevated serum creatinine (HR 2.83), hematocrit ≥ 43% (HR 1.05), and RA (vs OA; HR 1.79). Harrell’s c-index was 0.66 in the validation cohort and the model was well calibrated for the risk of major NSAID toxicity (calibration slope 0.75 for observed to predicted probabilities of outcomes). In the total population (n = 23,953), 1,389 (5.8%) had predicted 1 year risk <1%, 15,979 (66.7%) had predicted 1 year risk 1-4%, and 6,367 (26.6%) had predicted 1 year risk >4%. Calibration in the combined cohort is demonstrated in the survival probability plot (see Figure).
Conclusion: We have derived and internally validated a risk score using data from the PRECISION trial to predict the 1 year risk of major NSAID toxicity for patients with osteoarthritis or rheumatoid arthritis on chronic NSAIDs. Elements of the score are easy to obtain and, if found to be externally valid, could help clinicians and patients determine risk-benefits of chronic NSAID use.
To cite this abstract in AMA style:
Solomon D, Shao M, Wolski KE, Nissen SE, Husni ME, Paynter N. Major NSAID Toxicity: Derivation and Internal Validation of a Simple Clinical Risk Score [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/major-nsaid-toxicity-derivation-and-internal-validation-of-a-simple-clinical-risk-score/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/major-nsaid-toxicity-derivation-and-internal-validation-of-a-simple-clinical-risk-score/