Session Information
Session Type: Plenary Session
Session Time: 11:30AM-1:00PM
Background/Purpose: Rheumatoid arthritis (RA) patients (pts) in remission on combination therapy (Combo) of methotrexate (MTX)+etanercept (ETN) face ongoing medication burden and long-term safety/tolerability concerns related to continuing therapies. Reducing therapy has been studied, but whether pts could discontinue either MTX or ETN and maintain remission on monotherapy (mono) has not been rigorously tested. This study compared withdrawing either MTX or ETN on remission maintenance in RA pts who had been in sustained, stringent remission while on Combo.
Methods: The Study of ETN And MTX in RA (SEAM-RA) enrolled adult RA pts on ETN 50 mg/week + MTX 10-25 mg/week who met ACR/EULAR remission criteria (Simplified Disease Activity Index [SDAI] score ≤3.3) in a 24-week, open-label period. After 24 weeks, pts remaining in remission entered a 48-week, double-blind period and were randomized to: (1) withdrawal of ETN (MTX mono); (2) withdrawal of MTX (ETN mono); or (3) continue Combo. Pts with disease-worsening (DW; defined as SDAI >11 at any time or SDAI >3.3 and ≤11 on 2 consecutive visits ≥2 weeks apart or on ≥3 separate visits) received Combo rescue therapy (Combo arm continued Combo therapy) and were considered non-responders. Endpoints included proportion of pts in SDAI remission without DW at week 48 in the ETN mono vs MTX mono arms (primary) and in the Combo vs MTX mono arms (secondary). Other secondary endpoints included time to DW and time to recapture SDAI remission in pts needing rescue therapy. For the primary endpoint, non-responder imputation was used for missing data. The P-value for the primary endpoint was estimated from the Chi-squared test. For secondary endpoints, P-values were not adjusted.
Results: Of 371 pts enrolled in the 24-week, open-label period, 253 (68.2%) remained in remission and were randomized to the double-blind period (101 MTX mono, 101 ETN mono, and 51 Combo). Baseline values were similar across treatment arms: mean (SD) age 55.6 (12.2) years, RA duration 10.3 (7.8) years, MTX dose 16.3 (4.7) mg/week, and SDAI score of 1.3 (1.2). At week 48, SDAI remission was maintained by significantly more pts on ETN mono vs MTX mono (49.5% vs 28.7%; P=0.004) and by more pts on Combo vs MTX mono (52.9% vs 28.7%; P=0.006). Time to DW was shorter with MTX mono compared with ETN mono or Combo (P< 0.001 for both comparisons; Fig. 1). In pts with DW treated with Combo rescue therapy, the cumulative percentage who recaptured SDAI remission by end of study was 71%, 75%, and 80% in the MTX mono, ETN mono, and Combo arms, respectively. Time to recapture SDAI remission after initiating rescue therapy was similar in all 3 treatment arms. No new safety signals were reported.
Conclusion: In pts in remission on Combo who then withdrew either MTX or ETN, this study showed that ETN mono was superior to MTX mono in maintaining remission. Similar proportions of pts maintained remission with ETN mono as with Combo. The majority of pts who received rescue therapy recaptured remission. For pts and physicians seeking to reduce treatment burden, these data inform decision-making on therapy withdrawal in well-controlled RA pts.
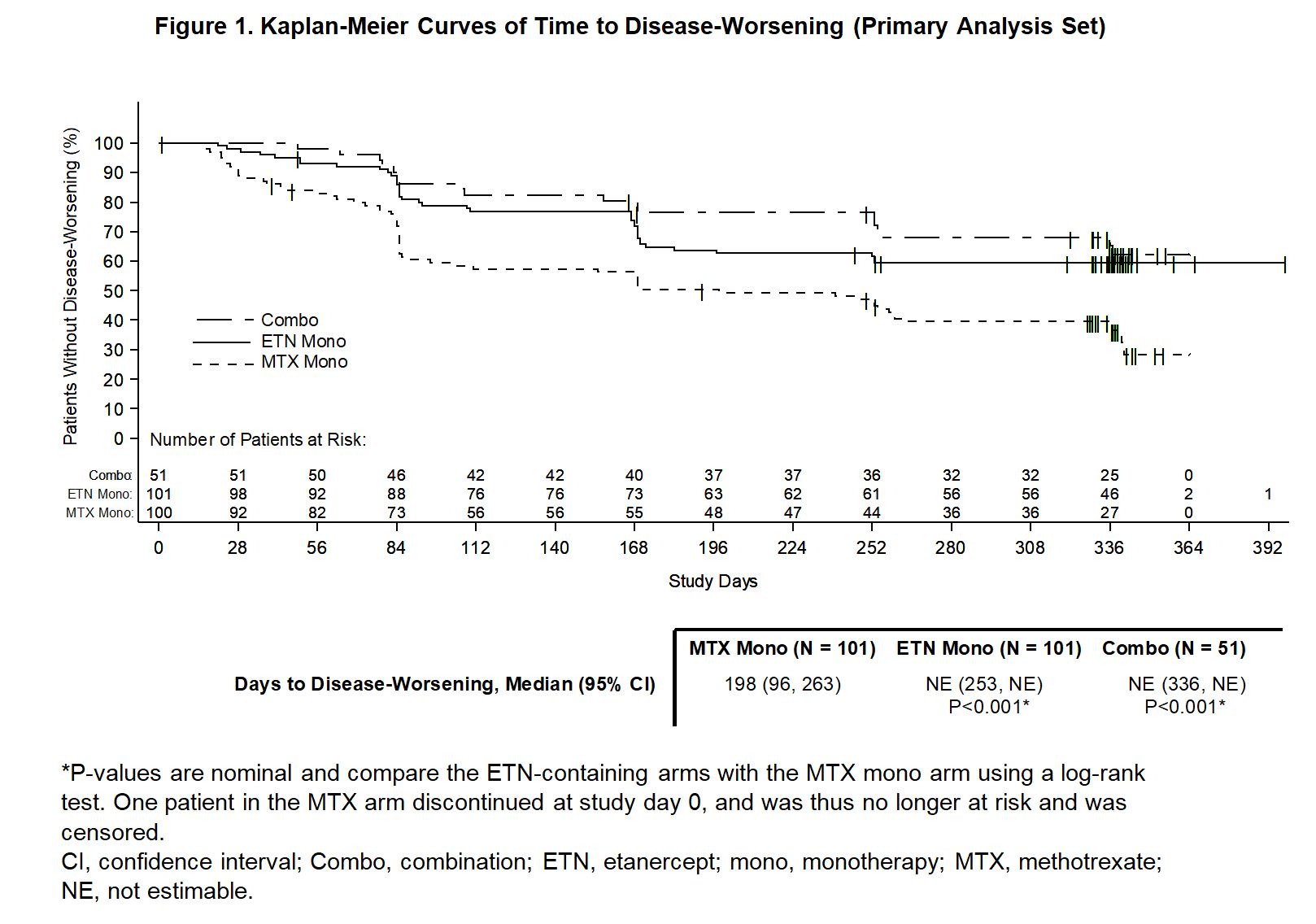 Figure 1. Kaplan-Meier Curves of Time to Disease-Worsening (Primary Analysis Set)
Figure 1. Kaplan-Meier Curves of Time to Disease-Worsening (Primary Analysis Set)
To cite this abstract in AMA style:
Curtis J, Emery P, Karis E, Haraoui B, Bykerk V, Yen P, Kricorian G, Chung J. Maintenance of Remission After Withdrawal of Etanercept or Methotrexate in Patients with Rheumatoid Arthritis in Sustained Remission on Combination Therapy: Results from a Randomized, Double-blind, Controlled Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/maintenance-of-remission-after-withdrawal-of-etanercept-or-methotrexate-in-patients-with-rheumatoid-arthritis-in-sustained-remission-on-combination-therapy-results-from-a-randomized-double-blind-co/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maintenance-of-remission-after-withdrawal-of-etanercept-or-methotrexate-in-patients-with-rheumatoid-arthritis-in-sustained-remission-on-combination-therapy-results-from-a-randomized-double-blind-co/
