Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The most commonly used intra-articular (IA) corticosteroid for osteoarthritis (OA) is triamcinolone acetonide (TA), but its potential chondrotoxicity restricts injection frequency to 3-4 injections per year. TLC599 is a liposomal formulation of dexamethasone sodium phosphate (DSP) designed for intra-articular injection in the treatment of knee osteoarthritis (OA). Based on a Phase 2a multi-center, randomized, double-blind, placebo-controlled clinical trial (ClinicalTrials.gov ID: NCT03005873), TLC599 has demonstrated durable pain relief and improved function over 24 weeks in patients with knee OA pain. Also, TLC599 possess the potential to protect articular cartilage from damage, making it potentially a beneficial therapy for OA with less risk of chondrotoxicity. To evaluate the effect of TLC599 on knee cartilage, magnetic resonance imaging (MRI) was utilized in this trial.
Methods: In the current Phase 2a study, subjects were eligible if they met the American College of Rheumatology Criteria for knee OA, with Grade 2 to 3 Kellgren-Lawrence severity. A total of 75 patients were randomized to receive 1 of the 3 regimens in the index knee in 1:1:1 ratio (TLC599 with 12 mg DSP and 100 μmol phospholipid (PL); TLC599 18 mg DSP with 150 μmol PL; or normal saline placebo). Patients were followed 24 weeks post injection. MRI was conducted on both knees before dosing and at the end of study, and cartilage was evaluated by a blinded radiologist at each center using the MRI Osteoarthritis Knee Score (MOAKS) instrument, with 14 articular subregions individually evaluated with 2 categorical scores, indicating the size of cartilage damage (surface area loss) and depth of cartilage damage (full thickness loss).
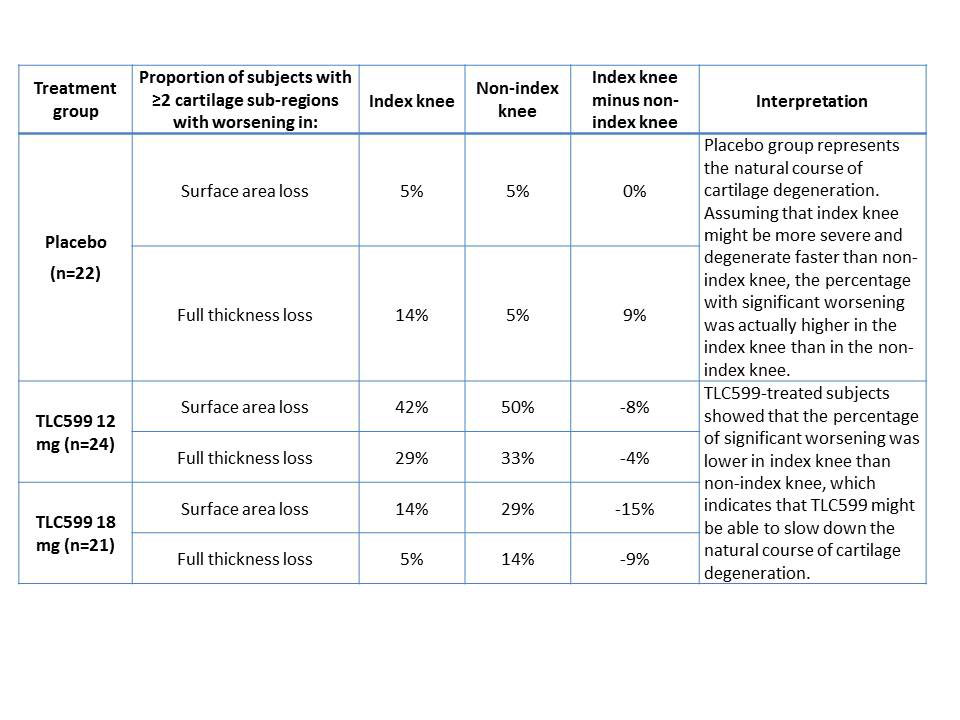
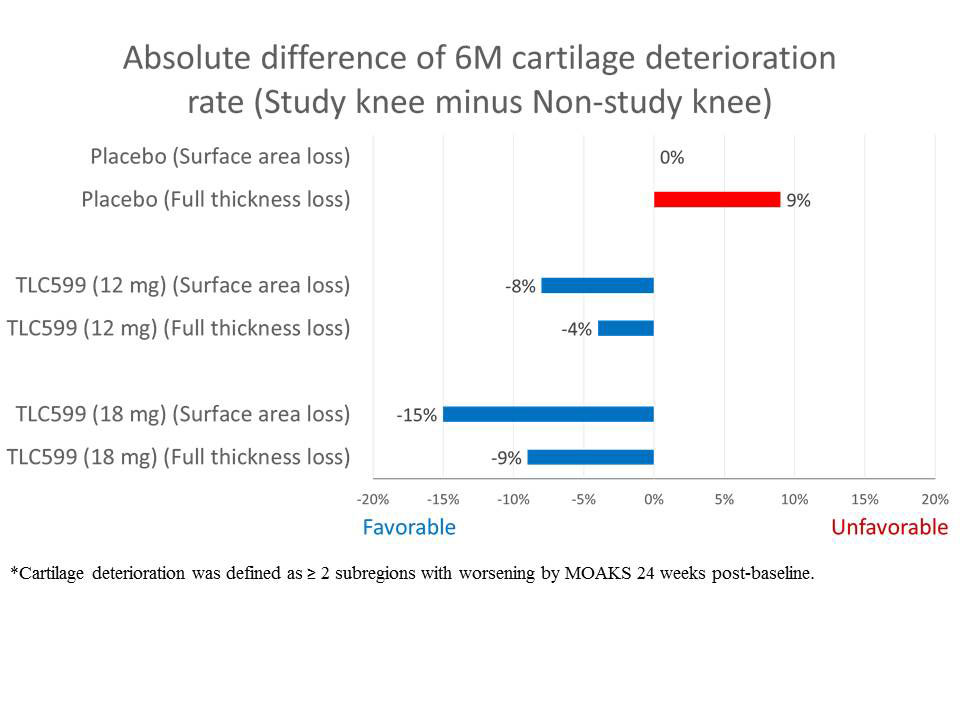
Results: Index knees were compared with non-index knees by number of sub-regions with worsening from baseline to the end of 24 weeks (≥ 2 sub-regions worsening representing significant worsening), regardless of the individual sub-region severity. Table 1 shows the MOAKS score pattern for placebo group index and non-index knees, representing the natural course of the disease, and the TLC599 group knees. For the placebo group, the index knee displayed similar or more significant cartilage damage (≥ 2 sub-regions with worsening) than the non-index knee, for both joint cartilage surface area and thickness. However, both TLC599 patient groups displayed lower proportions of significant cartilage damage in the index knee than the non-index knee. Figure 1 shows that the pattern of knee joint cartilage damage change between treated groups was reflected by the difference between the proportions of subjects displaying ≥2 worsening cartilage sub-regions in index vs. non-index knee. These findings suggest there may be protection from cartilage degeneration by TLC599 IA injection.
Conclusion: The exploratory MRI evaluation of knee cartilage using semi-quantitative MOAKS indicated that, comparing index to non-index knees, patients treated with TLC599 IA injection displayed relatively less cartilage loss in the treated knee than placebo patients, suggesting a lack of cartilage damage or potentially a chondroprotective effect.
To cite this abstract in AMA style:
Shih S, Brown C, Tai T, Chuang W. Magnetic Resonance Imaging of Knee Joint Protection Following an Intra-Articular Injection of Lipid-Based Dexamethasone Sodium Phosphate Sustained Release Formulation on Subjects with Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/magnetic-resonance-imaging-of-knee-joint-protection-following-an-intra-articular-injection-of-lipid-based-dexamethasone-sodium-phosphate-sustained-release-formulation-on-subjects-with-knee-osteoarthri/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/magnetic-resonance-imaging-of-knee-joint-protection-following-an-intra-articular-injection-of-lipid-based-dexamethasone-sodium-phosphate-sustained-release-formulation-on-subjects-with-knee-osteoarthri/