Session Information
Date: Tuesday, November 12, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster III: Comorbidities
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The risk of lymphoma is higher among patients with rheumatoid arthritis (RA) compared to general populations 1. Methotrexate (MTX), the anchor drug for RA, is considered to be associated with development of lymphoproliferative disorders (LPD) 2. Although a substantial proportion of the cases are reported from Japan, a large-scale epidemiological study on LPD in patients with RA has not been conducted. Thus, the purpose of this study is to investigate epidemiological and clinical characteristics, and risk factors of LPD in Japanese patients with RA.
Methods: This is a multi-institutional retrospective observational study. We enrolled patients who were aged 20 or older, met the 1987 American College of Rheumatology / European League against Rheumatism classification criteria for RA, and visited the registered hospitals for rheumatology training program of Japan College of Rheumatology at least once between 1st April 2011 and 31st July 2011. We excluded patients who had been diagnosed as having lymphoma before the enrollment. The first visit of each patient during the above 4 months was defined as the start of the observation with a follow-up period of 3 years onward. A patient who fulfilled the definition of LPD during the observation were followed for up to 5 years from the onset of LPD. LPD of this study included lymphoma with pathological diagnosis, LPD other than lymphoma with pathological diagnosis, and clinical LPD without pathological diagnosis. We calculated proportion of patients with LPD, and investigated risk factors for LPD using a logistic regression model, and described pathological features, vital prognosis, and cause of death in patients with LPD. We used a REDCap toll for collecting data.
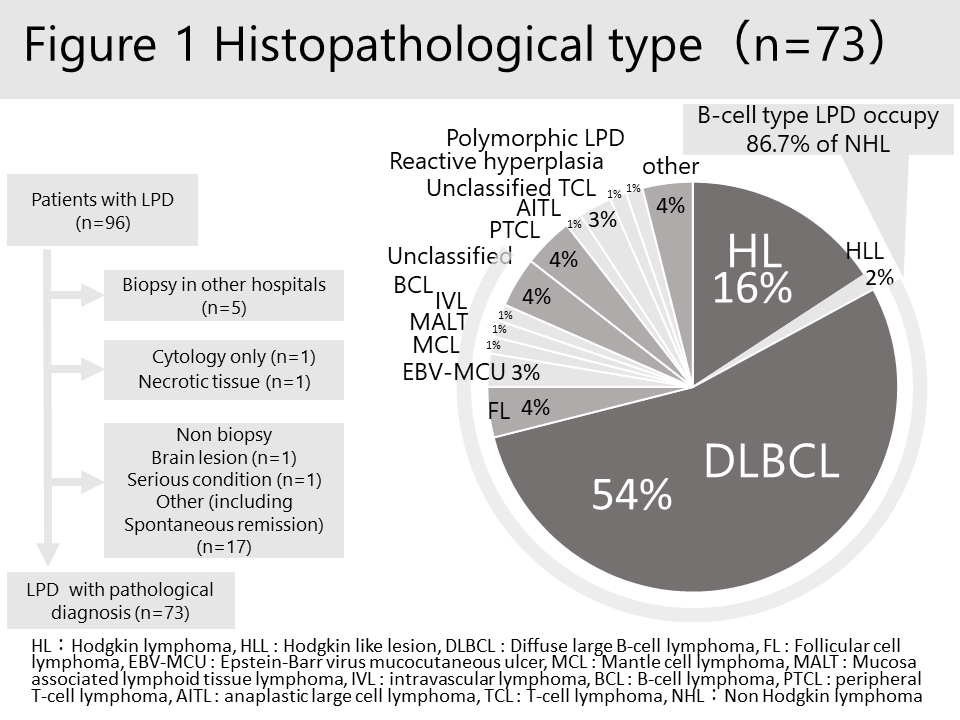
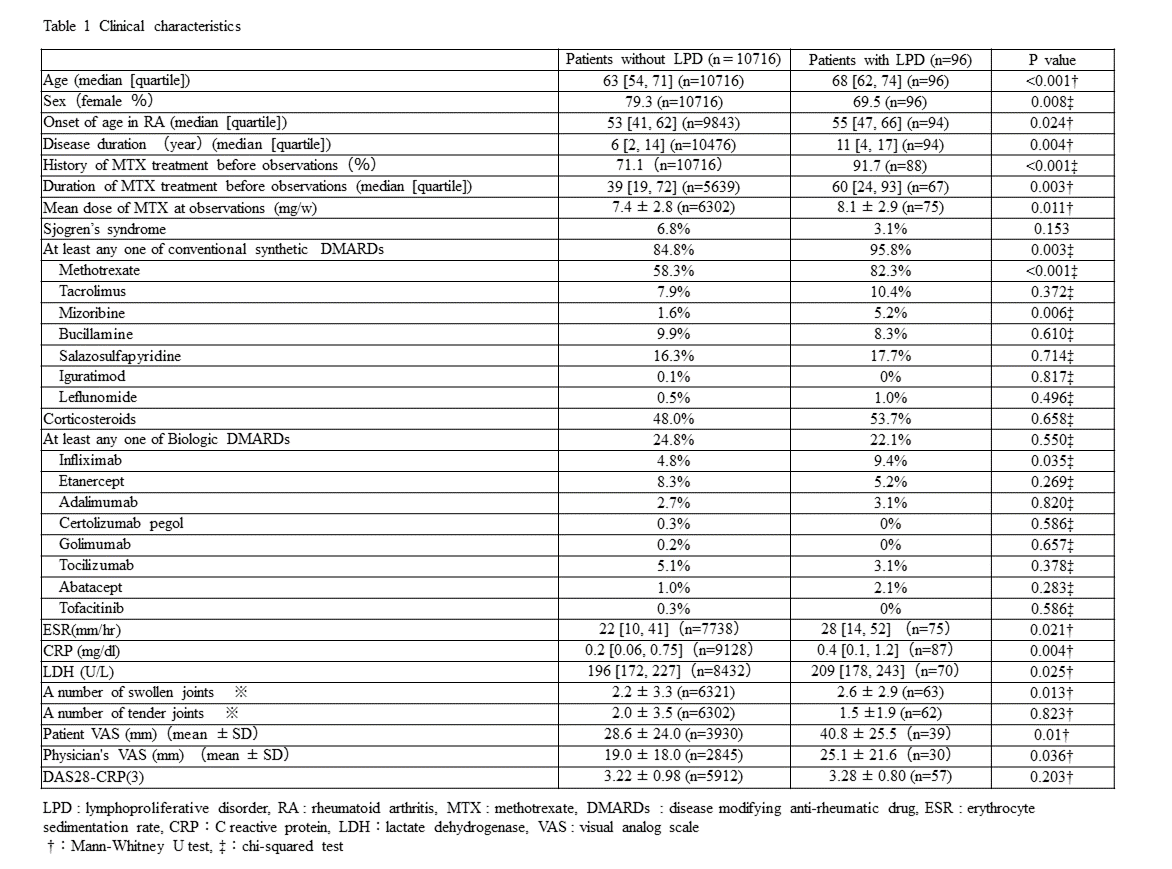
Results: Of 11100 patients enrolled, 10812 were analyzed. At baseline, the mean age was 63 years old and 79.3% were female. The mean disease duration was 9.8 years, 6.8% of the patients had Sjogren’s syndrome, and mean DAS28-CRP (3) was 3.22. Prevalence of MTX use and biological agent use was 58.6% and 22.8 %, respectively. The mean dose of MTX at baseline was 7.4 mg/week. Ninety-six patients (0.89%) developed LPD during the observation period. There were significant differences in patients’ characteristics such as age, sex, disease duration, and use of MTX between those who did and did not develop LPD (Table 1). Multivariable logistic regression analysis showed that age by decade (odds ratio [OR] 1.55 [95% CI 1.29-1.88]) and MTX use at baseline (3.40 [1.98-5.84] were significant independent risk factors of developing LPD (Table 2). Of 73 cases with pathological diagnosis, diffuse large B cell lymphoma (DLBCL) was the most frequent (n = 41, 54%), followed by Hodgkin lymphoma (n=12, 16%) (Figure 1). Mortality rate was 20% (n=23/92). The major cause of death was lymphoma (n=18, 78.3%), followed by cardiovascular diseases (n=2, 8.7 %).
Conclusion: Conclusions: This nationwide study revealed risk factors and pathological subtypes of LPD, and vital prognosis in Japanese patients with RA for the first time.
References
- Arthritis Res Ther. 2015; 17: 212.
- Am J Hematol. 2007; 82: 1106-9.
To cite this abstract in AMA style:
Honda S, Sakai R, Majima M, Konda N, Takada H, Yamamoto K, Takeuchi T, Harigai M. Lymphoproliferative Disorders in Patients with Rheumatoid Arthritis: Results from Japanese Multi-institutional Study Using Research Electronic Data Capture [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/lymphoproliferative-disorders-in-patients-with-rheumatoid-arthritis-results-from-japanese-multi-institutional-study-using-research-electronic-data-capture/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lymphoproliferative-disorders-in-patients-with-rheumatoid-arthritis-results-from-japanese-multi-institutional-study-using-research-electronic-data-capture/