Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Belimumab (BLM) is a B-cell stimulating factor (BlyS) monoclonal antibody, approved in 2022 for the treatment of lupus nephritis (NL) and since 2011 for systemic lupus erythematosus (SLE).
Objectives. To describe the demographic characteristics, efficacy and safety of BLM in real clinical practice in patients with lupus nephritis.
Methods: A descriptive, retrospective study of patients with lupus nephritis confirmed by biopsy from the Spanish multicenter registry of patients treated with BLM. Demographic, analytical and previous and concomitant treatment with belimumab were collected. To assess efficacy, analytical variables (anti-DNA, C3, C4), Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scale, and steroid doses at baseline and throughout follow-up were analyzed. Renal improvement was considered when a 24-hour proteinuria of less than 0.5 grams was reached. Safety profile of BLM was analyzed.
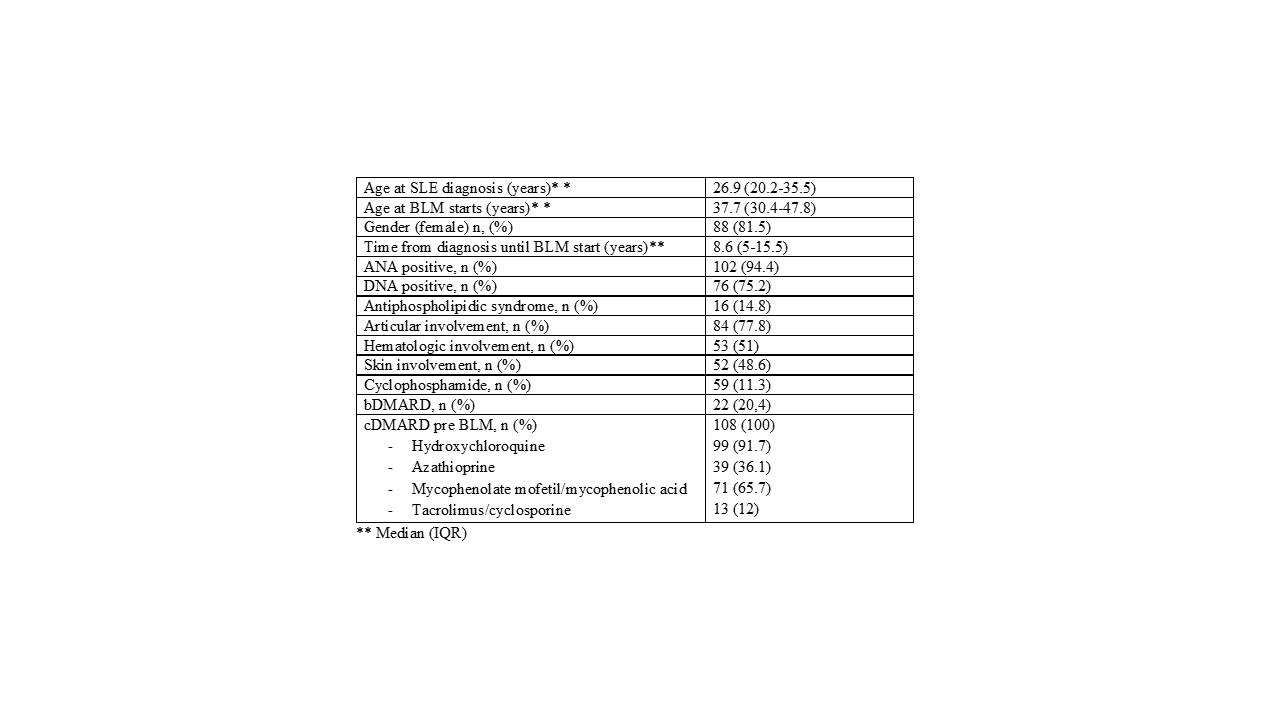
Results: We included 108 patients, 81.5% women, with a mean age at diagnosis of SLE of 26.9 years (20.2-35.5).The most frequent nephritis class was IV (47 patients, 43.5%), followed by class III (23 patients, 21.3%), V (19 patients, 17.6%) and I-II (19 patients, 17.6%). The time of evolution of the disease until the onset of BLM was 8.6 years (5-15.5).62 patients (57.4%) started BLM subcutaneously (SC), 33 patients (30.6%) intravenously (IV) and 13 patients (12%) change from IV to SC route. Baseline characteristics are summarized in Table 1
The median proteinuria in 24 hours was 1.0 (0.5 to 2.5). The median number of pre-BLM cDMARD uses was 2.0 (2.0-3.0), with antimalarials being the most commonly used (91.7%). 39 and 22 patients received cyclophosphamide and bDMARD prior to BLM respectively, being Rituximab (RTX) the most commonly bDMARD used. 107 patients received prednisone at the time of starting BLM with a median dose of 7.5 (5-1.5) mg.
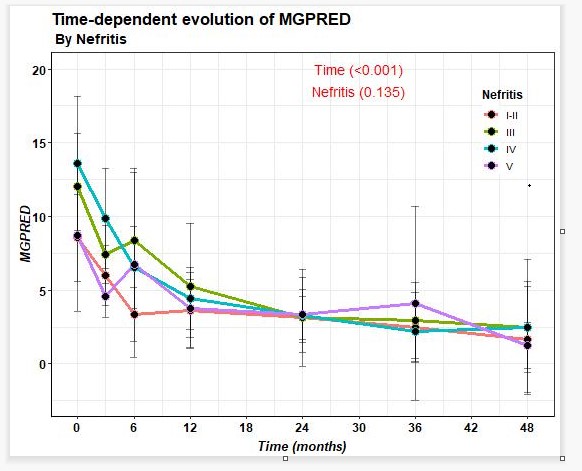
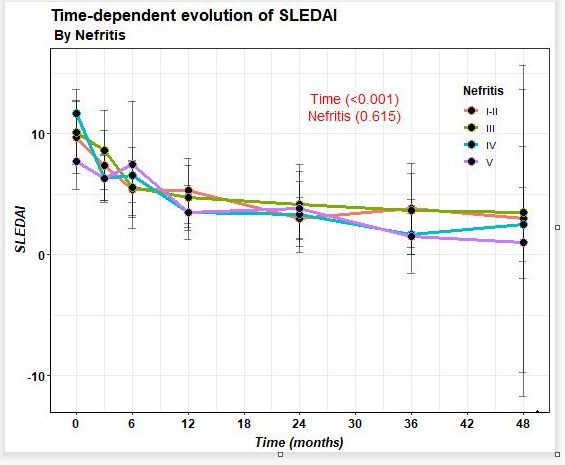
74.4% of patients improved after BLM in terms of reduction of proteinuria, with last 24-hour proteinuria of 0.2 (0.1-0.9) grams. A decrease in prednisone dose and SLEDAI improvement (Figure 1) was observed from baseline to last follow-up. On the other hand, a decrease in anti-DNA and an increase in C3 and C4 were observed.
21 patients (19.4%) discontinued treatment mainly due to ineffectiveness; 4 patients stopped BLM because of nephritis improvement. The median time on treatment for patients who had to discontinue BLM was 9 (6-24) months. In terms of safety, 28 patients had infections, most of them mild, being urine infection the most reported. One patient died due to meningitis. There were no tumors.
Conclusion: In this cohort of LN treated with BLM in real world settings, we observed an improvement of 24-hour proteinuria, SLEDAI, decrease of anti-DNA and increase of complement, despite of the administration route. No new safety alarms were reported.
To cite this abstract in AMA style:
laiño M, Enguita M, Navarro P, Loricera J, Garcia-Aparicio A, Lasa C, Calvo Río V, Gallego A, Moriano Morales C, Narvaez J, CARRION BARBERA M, Camins-Fàbregas J, Belzunegui Otano J, Urruticoechea A, del Olmo Perez L, Castañeda S, Quiroga P, Casafont-Sole I, Dios Jimenez De Aber J, López M, Font Urgelles J, Ortega Castro R, Garijo Bufort M, Fragio J, Vázquez I, Ortega M, Fariñas A, Peralta C, Blanco J, Heredia Martin S, Pinillos M, Labrador-Sánchez E, Cossio Jimenez P, Vazquez Galeano C, Aldasoro V. Lupus Nephritis of the Spanish National Registry of Belimumab in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/lupus-nephritis-of-the-spanish-national-registry-of-belimumab-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lupus-nephritis-of-the-spanish-national-registry-of-belimumab-in-patients-with-systemic-lupus-erythematosus/