Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: LN treatment goal is to suppress immune mediated injury and preserve eGFR. Studies indicate proteinuria < 700 at 12 months predicts maintaining eGFR at 7 or 10 years explaining the EULAR target. Proteinuria is reduced by effective immunosuppression (IS) or drugs that reduce proteinuria regardless of pathology(RASi, MRA, SGLT2i, or GLP1-RA). It is uncertain which patients at 1 year are candidates for repeat biopsy, intensified IS or additional anti-proteinuria drug(s). We hypothesize that these decisions will be assisted by assessing proteinuria and serological responses.
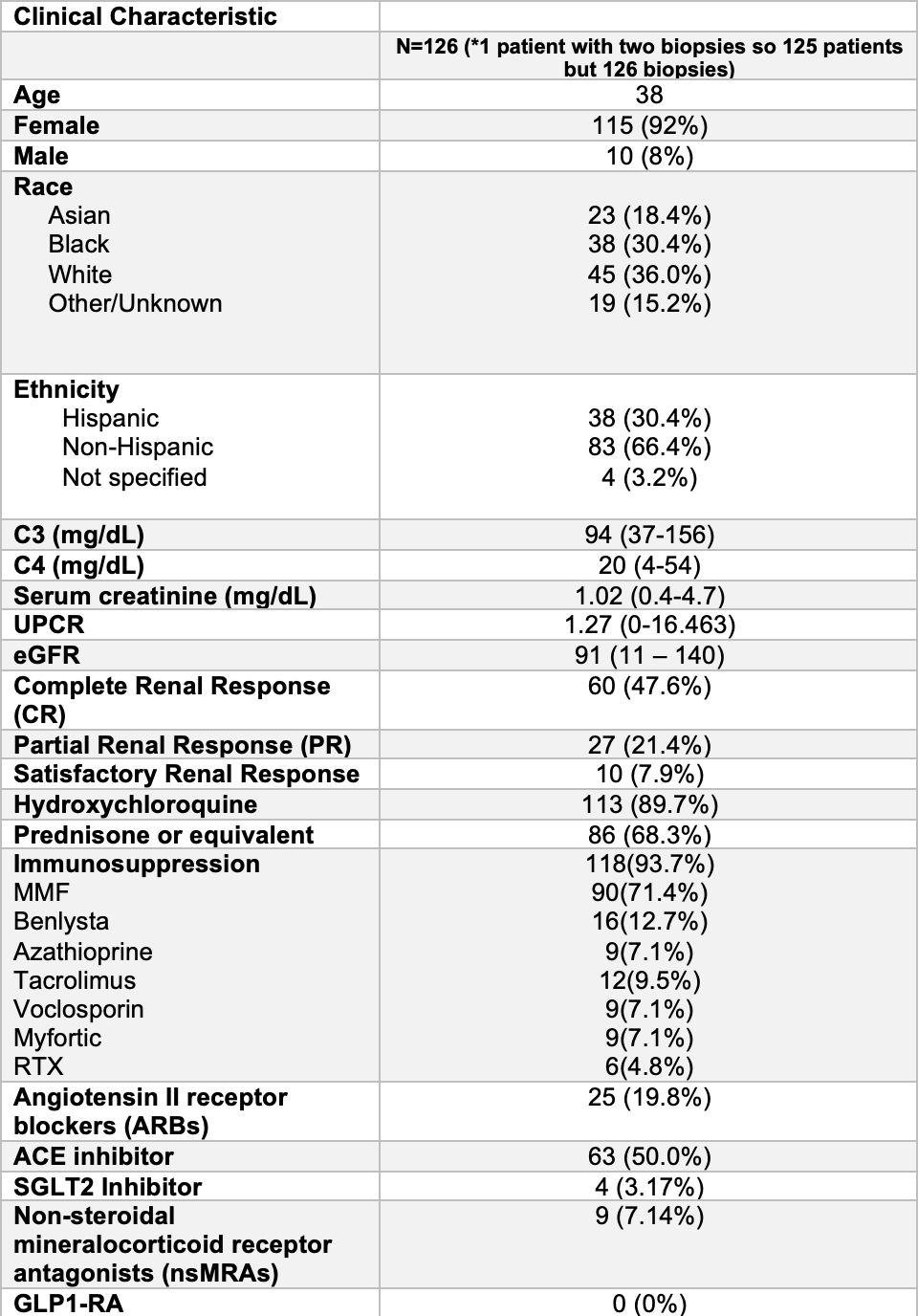
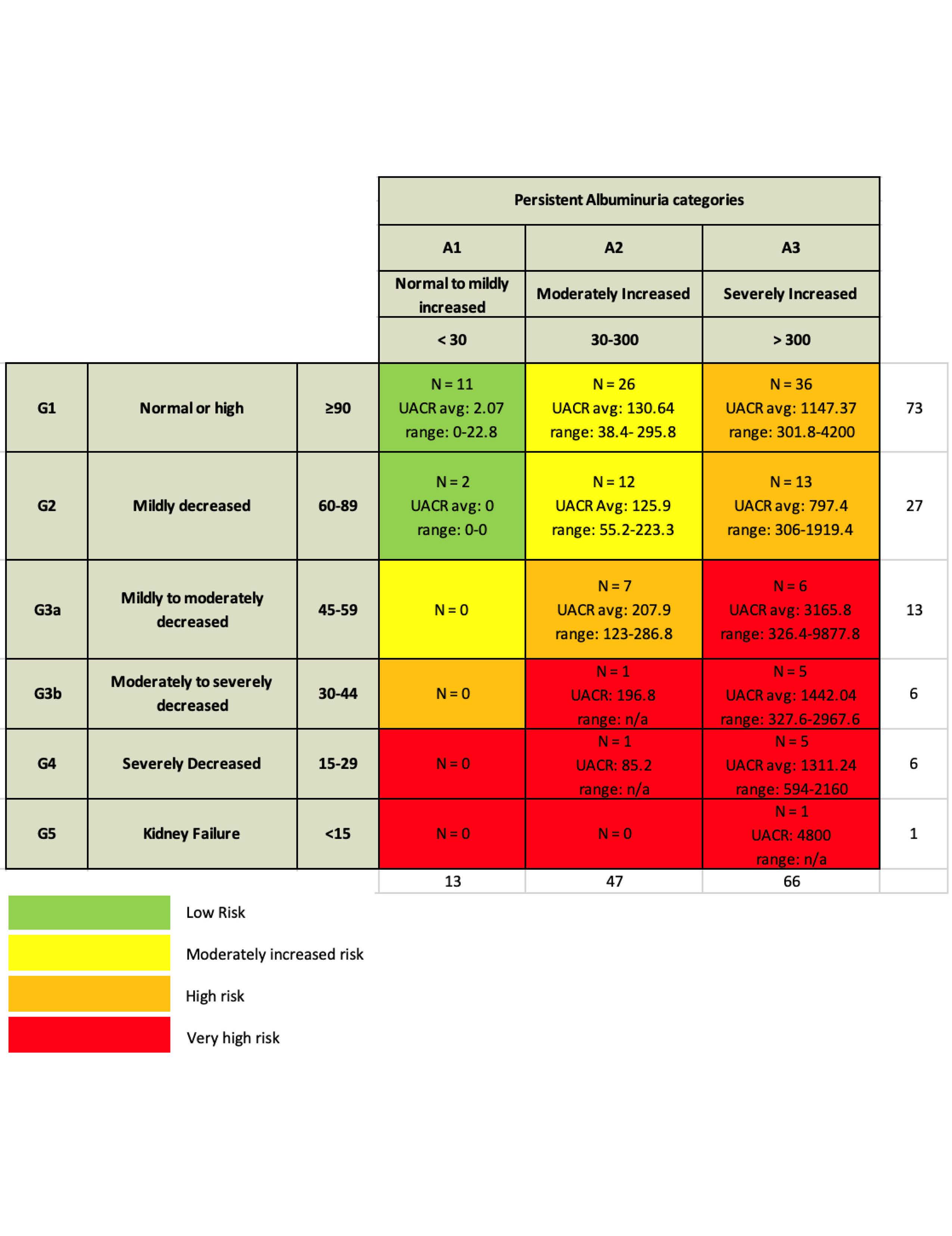
Methods: Identified patients in NYU SLE Registry with biopsy proven LN and data at 12 months after induction treatment 4/2017 – 4/2024. Data collected included demographics, dsDNA (DNA) by multiplex, C3/C4 by nephelometry, uPCR, CRR (uPCR< 500), SRR (uPCR< 700), PRR (uPCR< 50% baseline) and urine sediment. Relied on KDIGO 2012 heat map to determine CKD progression risk that categorizes magnitude of proteinuria relying on uACR, which we generated by using uPCR x 0.6, in association with eGFR.
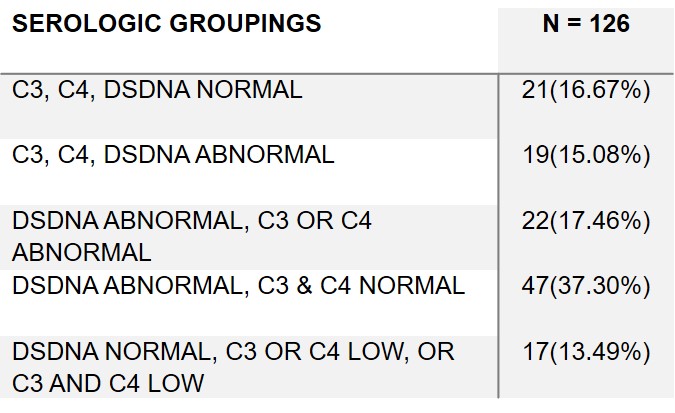
Results: 126 LN:115(92%) female, 45(36%) white, 38(30.4%) black, 23(18%) asian, and 38(30.4%) Hispanic Table 1. Only 21(16.7%) had a complete serological response with normalization of DNA and complement while 19(15.1%) had both elevated DNA and hypocomplementemia. Most frequent outcome was abnormal DNA but no hypocomplementemia in 47(37.3 %). In 22(17.5%) DNA elevated with low C3 or C4 but not both. In 17(13.5%) DNA normal with hypocomplementemia (low C3 or C4 or low C3 and C4)Table 2. 88(69.8%) on RASi, 4 (3.1%)SGL2i, 9(7.1%) MRA and 0 GLP1-RA Table 1. CRR achieved 60(47.6%), SRR 10(7.9%), and PRR 27(21.4%) Table 1. 103(81.7%) of patients had uACR > 300 and 47(37.3%) 30-300. 25(19.8%) patients uPCR > 700 and abnormal urine sediment. Based on proteinuria and eGFR only 13 patients had no risk for CKD progression, while 38 moderate, 56 high, and 19 very high risk Figure 1. 49 with either normal eGFR or only mildly decreased eGFR (60-89) at high risk due to magnitude of proteinuria. Very high risk in 12 based on serological activity and uPCR > 700. 25(19.8%) had uPCR > 700 and abnormal urine sediment. Another 9(7.1%) patients had normal serology and urine sediment, but uPCR > 700.
Conclusion: In a real world LN cohort complete serological responses are infrequent at 12 months. CRR, SRR or PRR are common and only 21.1% of patients without any clinical response defined by reduction in proteinuria. However, due to magnitude of proteinuria many remain at risk for CKD progression. Based on persistent serological activity or abnormal urine sediment and uPCR > 700 many patients are candidates for repeat biopsy to assess for suspected ongoing immune injury. Moreover, patients are eligible for anti-proteinuric medication based on normal serology and urine sediment, but uPCR > 700. Additional patients would be eligible for drug to reduce proteinuria if adopt stringent uACR goal, as in diabetic kidney disease, with target uACR < 300 or < 30, regardless of eGFR. Future data can clarify long term proteinuria target in patients with Grade 2 or worse CKD. At this time, clinical and serological responses at 12 months can clarify role of repeat biopsy, intensified IS, or drug for proteinuria reduction.
To cite this abstract in AMA style:
Belmont H, Cohen B, Saxena A, Izmirly P, Buyon J. Lupus Nephritis Clinical and Serological Responses at 12 Months: Implications for Repeat Biopsy, Intensified Immunosuppression or Additional Anti-proteinuric Therapy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/lupus-nephritis-clinical-and-serological-responses-at-12-months-implications-for-repeat-biopsy-intensified-immunosuppression-or-additional-anti-proteinuric-therapy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lupus-nephritis-clinical-and-serological-responses-at-12-months-implications-for-repeat-biopsy-intensified-immunosuppression-or-additional-anti-proteinuric-therapy/